Paper category: Original research paper
Corresponding author: Michal Adamski (michal.adamski@doctoral.uj.edu.pl)
DOI: 10.2478/ohs-2019-00020
Received: 25/01/2019
Accepted: 13/03/2019
Full text: here
Citation (APA style): Adamski, M., Żmudzki, P., Bialczyk, J., et al. (2019). Decomposition products of cylindrospermopsin – a cyanotoxin produced by Raphidiopsis raciborskii (Woloszynska). Oceanological and Hydrobiological Studies, 48(3), pp. 227-235. Retrieved 3 Oct. 2019, from doi:10.2478/ohs-2019-0020
Abstract
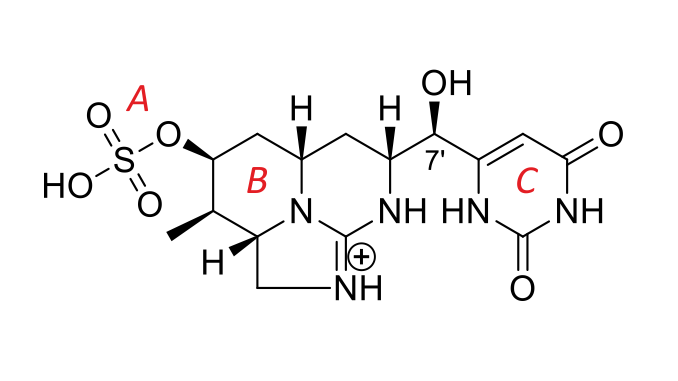
Toxins produced by cyanobacteria (cyanotoxins) and released into water have become a serious problem worldwide due to the increasing morbidity and mortality of living organisms they have caused. The ability to synthesize the cytotoxic alkaloid cylindrospermopsin (CYN) has been demonstrated in several freshwater species of cyanobacteria. CYN is highly chemically stable under environmental factors and decomposes only under alkaline conditions, where it forms derivatives. The toxicity potential of the decomposition products formed at pH 10 combined with high temperature (100°C) or UV-B irradiation (36 µmol m<sup>−2</sup> s<sup>−1</sup>) has been research based on the crustacean Thamnocephalus platyurus (Thamnotoxkit FTM) and bacteria Vibrio fischeri (Deltatox® II) bioassays. This paper is a continuation and completion of our previous experiments and the obtained results showed that the applied conditions contributed to the decomposition of the CYN molecule to non-toxic products and its structural modifications by separating the uracil ring or/and the sulfate group from the tricyclic guanidine moiety, leading to a reduction in its toxicity. To the best of our knowledge, this is the first report describing the toxicity of CYN decomposition products formed under alkaline conditions combined with boiling temperature or UV-B irradiation.
References
Adamski, M., Chrapusta, E., Bober, B., Kaminski, A. & Bialczyk, J. (2014). Cylindrospermopsin: cyanobacterial secondary metabolite. Biological aspects and potential risk for human health and life. Oceanol. Hydrobiol. St. 43: 442–449. DOI: 10.2478/s13545-014-0148-5.
Adamski, M., Żmudzki, P., Chrapusta, E., Bober, B., Kaminski, A. et al. (2016a). Effect of pH and temperature on stability of cylindrospermopsin. Characterization of decomposition products. Algal Research. 15: 129–134. DOI: 10.1016/j.algal.2016.02.020.
Adamski, M., Żmudzki, P., Chrapusta, E., Kaminski, A., Bober, B. et al. (2016b). Characterization of cylindrospermopsin decomposition products formed under irradiation conditions. Algal Research 18: 1–6. DOI: 10.1016/j.algal.2016.05.027.
An, J., Li, N., Wang, S., Liao, C., Zhou, L. et al. (2019). A novel electro-coagulation-Fenton for energy efficient cyanobacteria and cyanotoxins removal without chemical addition. J. Hazard. Mater. 5 March: 650–658.
Bober, B. & Bialczyk, J. (2017). Determination of the toxicity of the freshwater cyanobacterium Woronichinia naegeliana (Unger) Elenkin. J. Appl. Phycol. 29: 1355–1362. DOI: 10.1007/s10811-017-1062-1.
Bourke, A.T.C., Hawes, R.B., Neilson, A. & Stallman, N.D. (1983). An outbreak of hepato-enteritis (the Palm Island mystery disease) possibly caused by algal intoxication. Toxicon 21 Supple: 45–48. DOI: 10.1016/0041-0101(83)90151-4.
Carmichael, W.W. (2001). Health effects of toxin-producing cyanobacteria: “The Cyano-HABs”. Hum. Ecol. Risk. Assess. 7: 1393–1407.
Chiswell, R.K., Shaw, G.R., Eaglesham, G., Smith, M.J., Norris, R.L. et al. (1999). Stability of cylindrospermopsin the toxin from the cyanobacterium Cylindrospermopsis raciborskii: effect of pH temperature and sunlight on decomposition. Environ. Toxicol. 14: 155–161. DOI: 10.1002/(SICI)1522-7278(199902)14:13.0.CO;2-Z.
De la Cruz, A., Hiskia, A., Kaloudis, T., Chernoff, N., Hill, D. et al. (2013). A review on cylindrospermopsin: the global occurrence detection toxicity and degradation of a potent cyanotoxin. Environ. Sci. Process. Impacts 15: 1979–2003. DOI:10.1039/c3em00353a.
El-Sheikh, S.M., Khedr, T.M., Zhang, G., Vogiazi, V. Ismail, A.A. et al. (2017). Tailored synthesis of anatase–brookite heterojunction photocatalysts for degradation of cylindrospermopsin under UV–Vis light. Chem. Eng. J. 310: 428–436. DOI: 10.1016/j.cej.2016.05.007.
Everson, S., Fabbro, L., Kinnear, S., Eaglesham, G. & Wright, P. (2009). Distribution of the cyanobacterial toxins cylindrospermopsin and deoxycylindrospermopsin in a stratified lake in north-eastern New South Wales, Australia. Mar. Freshwater Res. 60: 25–33. DOI: 10.1071/MF08115.
Fabbro, L., Baker, M., Duivenvoorden, L., Pegg, G. & Shiel, R. (2001). The effects of the ciliate Paramecium cf. caudatum Ehrenberg on toxin producing Cylindrospermopsis isolated from the Fitzroy river Australia. Environ. Toxicol. 16: 489–497. DOI: 10.1002/tox.10007.
Falconer, I.R. & Humpage, A.R. (2001). Preliminary evidence for in vivo tumour initiation by oral administration of extracts of the blue-green alga Cylindrospermopsis raciborskii containing the toxin cylindrospermopsin. Environ. Toxicol. 16: 192–195. DOI: 10.1002/tox.1024.
Freitas, M., Azevedo, J., Carvalho, A.P., Mendes, V.M., Manadas, B. et al. (2016). Bioaccessibility and changes on cylindrospermopsin concentration in edible mussels with storage and processing time. Food Control 59: 567–574. DOI: 10.1016/j.foodcont.2015.06.025.
Froscio, S.M., Humpage, A.R., Wickramasinghe, W., Shaw, G. & Falconer, I.R. (2008). Interaction of the cyanobacterial toxin cylindrospermopsin with the eukaryotic protein synthesis system. Toxicon 51: 191–198. DOI: 10.1016/j.toxicon.2007.09.001.
Gao, Y., Cornwell, J.C., Stoecker, D.K. & Owens, M.S. (2012). Effects of cyanobacterial-driven pH increases on sediment nutrient fluxes and coupled nitrification-denitrification in a shallow fresh water estuary. Biogeosciences 9: 2697–2710. DOI: 10.5194/bg-9-2697-2012.
Guzmán-Guillén, R., Maisanaba, S., Prieto Ortega, A.I., Valderrama-Fernández, R., Jos, A. et al. (2017). Changes on cylindrospermopsin concentration and characterization of decomposition products in fish muscle (Oreochromis niloticus) by boiling and steaming. Food Control 77: 210–220. DOI: 10.1016/j.foodcont.2017.02.035.
Harada, K.I., Ohtani, I., Iwamoto, K., Suzuki, M., Watanabe, M.F. et al. (1994). Isolation of cylindrospermopsin from a cyanobacterium Umezekia natans and its screening method. Toxicon 32: 73–84. DOI: 10.1016/0041-0101(94)90023-X.
Humpage, A.R., Fontaine, F., Froscio, S., Burcham, P. & Falconer, I.R. (2005). Cylindrospermopsin genotoxicity and cytotoxicity role of cytochrome P-450 and oxidative stress J. Toxicol. Environ. Health. A. 68: 739–753. DOI: 10.1080/15287390590925465.
Hsieh, C.Y., Tsai, M.H., Ryan, D.K. & Pancorbo, O.C. (2014). Toxicity of the 13 priority pollutant metals to Vibrio fisheri in the Microtox® chronic toxicity test. Sci. Total. Environ. 320: 37–50. DOI: 10.1016/S0048-9697(03)00451-0.
Klitzke, S., Apelt, S., Weiler, C., Fastner, J. & Chorus, I. (2010). Retention and degradation of the cyanobacterial toxin cylindrospermopsin in sediments – The role of sediment preconditioning and DOM composition. Toxicon 55: 999–1007. DOI: 10.1016/j.toxicon.2009.06.036.
Klitzke, S. & Fastner, J. (2012). Cylindrospermopsin degradation in sediments – The role of temperature redox conditions and dissolved organic carbon. Water Res. 46: 1549–1555. DOI: 10.1016/j.watres.2011.12.014.
Kubickova, B., Laboha, P., Hildebrandt, J., Hilscherová, K. & Babica, P. (2019). Effects of cylindrospermopsin on cultured immortalized human airway epithelial cells. Chemosphere 220: 620–628. DOI: 10.1016/j.chemosphere.2018.12.157.
López-Alonso, H., Rubiolo, J.A., Vega, F., Vieytes, M.R. & Botana, L.M. (2013). Protein synthesis inhibition and oxidative stress induced by cylindrospermopsin elicit apoptosis in primary rat hepatocytes. Chem. Res. Toxicol. 26: 203–212. DOI: 10.1021/tx3003438.
Meriluoto, J. & Codd, G.A. (2005) Toxic: Cyanobacterial Monitoring and Cyanotoxin Analysis. Åbo Akademi University Press, Åbo, Finland.
Metcalf, J.S., Lindsay, J., Beattie, K.A., Birmingham, S., Saker, M.L. et al. (2002). Toxicity of the cylindrospermopsin to the brine shrimp Artemia salina: comparisons with protein synthesis inhibitors and microcystins. Toxicon 40: 1115–1120. DOI: 10.1016/S0041-0101(02)00105-8.
Mohamed, Z.A. & Alamri, S.A. (2012). Biodegradation of cylindrospermopsin toxin by microcystin-degrading bacteria isolated from cyanobacterial blooms. Toxicon 60: 1390–1395. DOI: 10.1016/j.toxicon.2012.10.004.
Neumann, C. Bain, P. & Shaw, G. (2007). Studies of the comparative in vitro toxicology of the cyanobacterial metabolite deoxycylindrospermopsin. J. Toxicol. Environ. Health A. 70: 1679–1686. DOI: 10.1080/15287390701434869.
Ohtani, I. & Moore, R.E. (1992). Cylindrospermopsin: A potent hepatotoxin from the Blue-Green Alga Cylindrospermopsis raciborskii. J. Am. Chem. Soc. 114: 7941–7942. DOI: 10.1021/ja00046a067.
Pinheiro, C., Azevedo, J., Campos, A., Vasconcelos, V. & Loureiro, S. (2016). The interactive effects of microcystin-LR and cylindrospermopsin on the growth rate of the freshwater algae Chlorella vulgaris. Ecotoxicology 25: 745–758. DOI: 10.1007/s10646-016-1633-y.
Runnegar, M.T., Kong, S.M., Zhong, Y.Z. & Lu, S.C. (1995). Inhibition of reduced glutathione synthesis by cyanobacterial alkaloid cylindrospermopsin in cultured rat hepatocytes. Biochem. Pharmacol. 49: 219–225. DOI: 10.1016/S0006-2952(94)00466-8.
Saker, M.L. & Eaglesham, G.K. (1999) The accumulation of cylindrospermopsin from the cyanobacterium Cylindrospermopsis raciborskii in tissues of the Redclaw crayfish Cherax quadricarinatus. Toxicon 37: 1065–1077. DOI: 10.1016/S0041-0101(98)00240-2.
Saker, M.L., Metcalf, J.S., Codd, G.A. & Vasconcelos, V.M. (2004). Accumulation and depuration of the cyanobacterial toxin cylindrospermopsin in the freshwater mussel Anodonta cygnea. Toxicon 43: 185–194. DOI: 10.1016/j.toxicon.2003.11.022.
Santos, C., Azevedo, J., Campos, A., Vasconcelos, V. & Pereira, A.L. (2015). Biochemical and growth performance of the aquatic macrophyte Azolla filiculoides to sub-chronic exposure to cylindrospermopsin. Ecotoxicology 24: 1848–1857. DOI: 10.1007/s10646-015-1521-x.
Sierosławska, A. (2013). Evaluation of the sensitivity of organisms used in commercially available toxkits to selected cyanotoxins. Pol. J. Environ. Stud. 22: 1817–1823.
Spoof, L., Berg, K.A., Rapala, J., Lahti, K., Lepistö, L. et al. (2006). First observation of cylindrospermopsin in Anabaena lapponica isolated from the boreal environment (Finland). Environ. Toxicol. 21: 552–560. DOI: 10.1002/tox.20216.
Stanier, R.Y., Kunisawa, R., Mandel, M. & Cohen-Bazire, G. (1971). Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 35: 171–205.
Weirich, C.A. & Miller, T.R. (2014). Freshwater harmful algal blooms: toxins and children’s health. Curr. Probl. Pediatr. Adolesc. Health Care 44: 2–24. DOI: 10.1016/j.cppeds.2013.10.007.
Wörmer, L., Huerta-Fontela, M., Cirés, S., Carrasco, D. & Quesada, A. (2010). Natural photodegradation of the cyanobacterial toxins microcystin and cylindrospermopsin. Environ. Sci. Technol. 44: 3002–3007. DOI: 10.1021/es9036012.
Yan, S., Jia, A., Merel, S., Snyder, S.A., O’Shea, K.E. et al. (2016). Ozonation of cylindrospermopsin (cyanotoxin): degradation mechanisms and cytotoxicity assessments. Environ. Sci. Technol. 50: 1437–1446. DOI: 10.1021/acs.est.5b04540.


Bądź pierwszy, który skomentuje ten wpis