Paper category: Original research paper
Corresponding author: Marcin Polonis (marcin.polonis@o2.pl)
DOI: 10.1515/ohs-2021-0015
Received: 14/09/2020
Accepted: 16/11/2020
Full text: here
Citation (APA style): Polonis,M.,Błaszczyk,A.,Jagiełło,K.,Panasiak,L.,Dobosz,S. & Ocalewicz,K.(2021).Inter-clutch egg differences and androgenesis in rainbow trout (Oncorhynchus mykiss, Walbaum 1792). Oceanological and Hydrobiological Studies,50(2) 160-168. https://doi.org/10.2478/oandhs-2021-0015
Abstract
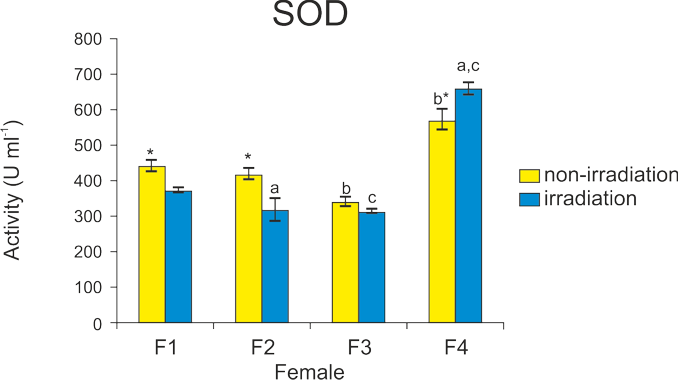
Ionizing radiation (IR) is applied to inactivate the nuclear genome in rainbow trout eggs during induced androgenetic development. However, IR-generated reactive oxygen species (ROS) may affect developmental potential of eggs and reduce the effectiveness of androgenesis. To verify this assumption, androgenetic development of rainbow trout was induced in eggs irradiated with 350 Gy of X-rays. Survival rates, pH of the ovarian fluid and activity of antioxidant enzymes, including SOD, CAT and GPx, were examined in non-irradiated and irradiated eggs originating from four females. Survival rates of androgenetic embryos developing in eggs produced by different females varied from 1% to 57% and these inter-clutch differences were significant. Eggs from female F4, which showed the highest developmental competence for androgenesis, also showed increased activities of SOD, CAT and GPx enzymes. The pH value of the ovarian fluid of each female was over 8 before and after irradiation, therefore it seems that radiation did not affect the ovarian fluid pH. Considering the above-mentioned inter-clutch differences, a strong maternal effect on the effectiveness of androgenesis can be assumed. Eggs with increased activity of antioxidant enzymes before irradiation should be expected to show increased developmental competence for androgenesis.
Conclusions
Eggs from four rainbow trout females used in the present experiment were characterized by similar quality. However, the survival of androgenetic specimens developing in these eggs varied significantly. There were also significant inter-clutch differences in the activity of the antioxidant enzymes. Patterns of SOD, CAT and GPx activity were different in non-irradiated eggs and eggs exposed to X-rays for androgenesis. However, the effect of X-rays was minor. The highest survival rate of androgenetic specimens was observed in eggs that showed increased CAT and GPx activities before irradiation. The high inter-clutch variation in the enzyme activity and survival of androgenetic progeny suggested the involvement of maternal genetic factor(s) enabling the effective development of androgenetic trout. There was no significant effect of X-rays on the acid-base balance of the ovarian fluid, which was considered important for the survival of androgenotes.
Acknowledgements
This work was supported by the University of Gdańsk, Poland (grant number 538-G205-B108-18). We thank Rafał Różyński from the Department of Salmonid Research, Inland Fisheries Institute in Olsztyn, Rutki, for his technical assistance during the experiment.
References
Aegerter, S. & Jalabert, B. (2004). Effects of post-ovulatory oocyte ageing and temperature on egg quality and on the occurrence of triploid fry in rainbow trout, Oncorhynchus mykiss. Aquaculture 231(1-4): 59–71. DOI: 10.1016/j.aquaculture.2003.08.019.
Babiak, I. Dobosz, S. Goryczko, K. Kuzminski, H. Brzuzan, P. et al. (2002). Androgenesis in rainbow trout using cryopreserved spermatozoa: the effect of processing and biological factors. Theriogenology 57(4): 1229–1249. DOI: 10.1016/S0093-691X(02)00631-3.
Chatakondi, N.G. Torrans, E.L. (2012). The influence of ovarian fluid pH of stripped unfertilized channel catfish, Ictalurus punctatus, eggs on the hatching success of channel catfish♀ x blue catfish, Ictalurus furcatus♂, hybrid catfish eggs. J World Aquacult. Soc. 43(4): 585–593. DOI: 10.1111/j.1749-7345.2012.00577.x.
Ciereszko, A., Wojtczak, M., Dietrich, G.J., Kuźmiński, H. & Dobosz, S. (2009). A lack of consistent relationship between distribution of lipid droplets and egg quality in hatchery‐raised rainbow trout, Oncorhynchus mykiss. Aquaculture, 289(1–2): 150–153. DOI: 10.1016/j.aquaculture.2008.12.032.
Fopp-Bayat, D. & Ocalewicz, K. (2015). Activation of the albino sterlet Acipenser ruthenus eggs by UV-Irradiated bester hybrid spermatozoa to provide gynogenetic progeny. Reprod. Domest. Anim. 50(4): 554–559. DOI: 10.1111/rda.12521.
Goud, A.P. Goud, P.T. Diamond, M.P. Gonik, B. & Abu-Soud, H.M. (2008). Reactive oxygen species and oocyte aging: role of superoxide, hydrogen peroxide, and hypochlorous acid. Free Radical Bio. Med. 44(7): 1295–1304. DOI: 10.1016/j.freeradbiomed.2007.11.014.
Gurgul, A., Pawlina-Tyszko, K., Bugno-Poniewierska, M., Szmatoła, T., Jasielczuk, I. et al. (2018). Transcriptome analysis of rainbow trout (Oncorhynchus mykiss) eggs subjected to the high hydrostatic pressure treatment. Int. J. Genomics. DOI: 10.1155/2018/5197126.
Ismail, N.A. Okasha, S.H. Dhawan, A. Rahman, A.M.A. Hamid, N.A. et al. (2012). Glutathione peroxidase, superoxide dismutase and catalase activities in children with chronic hepatitis. DOI: 10.4236/abb.2012.327119.
Jagiełło, K. Dobosz, S. Zalewski, T. Polonis, M. & Ocalewicz, K. (2018). Developmental competence of eggs produced by rainbow trout Doubled Haploids (DHs) and generation of the clonal lines. Reprod. Domest. Anim. 53(5): 1176–1183. DOI: 10.1111/rda.13223.
Komen, H. & Thorgaard, G. (2007). Androgenesis, gynogenesis and the production of clones in fishes: A review. Aquaculture 269: 150–173. DOI: 10.1016/j.aquaculture.2007.05.009.
Lahnsteiner, F. (2000). Morphological, physiological and biochemical parameters characterizing the over-ripening of rainbow trout eggs. Fish. Physiol. Biochem. 23(2): 107–118. DOI: 10.1023/A:1007839023540.
Mansour, N. Lahnsteiner, F. & Patzner, R.A. (2007). Distribution of lipid droplets is an indicator for egg quality in brown trout, Salmo trutta fario. Aquaculture 273(4): 744–747. DOI: 10.1016/j.aquaculture.2007.09.027.
Mantovani, G., Macciò, A., Madeddu, C., Mura, L. Gramignano, G. et al. (2003). The impact of different antioxidant agents alone or in combination on reactive oxygen species, antioxidant enzymes and cytokines in a series of advanced cancer patients at different sites: correlation with disease progression. Free Radical Res. 37(2): 213–223. DOI: 10.1080/10715760303849.
Michalik, O., Dobosz, S., Zalewski, T., Sapota, M. & Ocalewicz, K. (2015). Induction of gynogenetic and androgenetic haploid and doubled haploid development in the brown trout (Salmo trutta Linnaeus 1758). Reprod. Domest. Anim. 50(2): 256–262. DOI: 10.1111/rda.12480.
Migaud, H., Bell, G., Cabrita, E., McAndrew, B., Davie, A. et al. (2013). Gamete quality and broodstock management in temperate fish. Rev. Aquacult. 5: 194–223. DOI: 10.1111/raq.12025.
Ocalewicz, K., Gurgul, A., Pawlina-Tyszko, K., Szmatoła, T., Jasielczuk, I. et al. (2019). Induced androgenetic development in rainbow trout and transcriptome analysis of irradiated eggs. Sci. Rep-UK 9(1): 1–12. DOI: 10.1038/s41598-019-44568-7.
Ocalewicz, K., Gurgul, A., Polonis, M. & Dobosz, S. (2020). Preliminary Identification of Candidate Genes Related to Survival of Gynogenetic Rainbow Trout (Oncorhynchus mykiss) Based on Comparative Transcriptome Analysis. Animals 10(8): 1326. DOI: 10.3390/ani10081326.
Ocalewicz, K., Kuzminski, H., Pomianowski, K. & Dobosz, S. (2013). Induction of androgenetic development of the brook charr (Salvelinus fontinalis)× Arctic charr (Salvelinus alpinus) hybrids in eggs derived from the parental species. Reprod. Biol. 13(2): 105–112. DOI: 10.1016/j.repbio.2013.03.002.
Pandian, T.A. & Koteeswaran, R. (1998). Ploidy induction and sex control in fish. Hydrobiologia 384(1–3): 167–243. DOI: 10.1023/A:1003332526659.
Polonis, M., Jagiełło, K., Dobosz, S., Rożyński, R., Kondraciuk, P. et al. (2019). Alterations in the rainbow trout (Oncorhynchus mykiss) eggs exposed to ionizing radiation during induced androgenesis. Reprod. Domest. Anim. 54(4): 712–718. DOI: 10.1111/rda.13413.
Prieto, A.I., Jos, Á., Pichardo, S., Moreno, I. & Cameán, A.M. (2006). Differential oxidative stress responses to microcystins LR and RR in intraperitoneally exposed tilapia fish (Oreochromis sp.). Aquat. Toxicol. 77(3): 314–321. DOI: 10.1016/j.aquatox.2005.12.012.
Rauwerda, H., Wackers, P., Pagano, J.F., de Jong, M., Ensink, W. et al. (2016). Mother-specific signature in the maternal transcriptome composition of mature, unfertilized zebrafish eggs. PLoS One 11(1). DOI: 10.1371/journal.pone.0147151.
Rexroad, C.E. & Palti, Y. (2003). Development of ninety-seven polymorphic microsatellite markers for rainbow trout. Trans. Am. Fish. Soc. 132(6): 1214–1221 DOI: 10.1577/T02-086.
Rexroad, C.E., Coleman, R.L., Hershberger, W.K. & Killefer, J. (2002). Rapid communication: Thirty-eight polymorphic microsatellite markers for mapping in rainbow trout. Anim. Sci. J. 80: 541–542.
Rexroad, C.E., Coleman, R.L., Martin, A.M., Hershberger, W.K. & Killefer, J. (2001). Thirty-five polymorphic microsatellite markers for rainbow trout (Oncorhynchus mykiss). Anim. Genet. 32(5): 317–319.
Samarin, A.M., Samarin, A.M., Østbye, T-K.K., Ruyter, B., Sampels, S. et al. (2019a). Alteration of mRNA abundance, oxidation products and antioxidant enzyme activities during oocyte ageing in common carp Cyprinus carpio. PloS One 14(2): DOI: 10.1371/journal.pone.0212694.
Samarin, A.M., Samarin, A.M., Østbye, T-K.K., Ruyter, B., Sampels, S. et al. (2019b). The possible involvement of oxidative stress in the oocyte ageing process in goldfish Carassius auratus (Linnaeus, 1758). Scientific reports 9(1): 1–9. DOI: 10.1038/s41598-019-46895-1.
Samarin, A.M., Samarin, A.M. & Policar, T. (2018a). Cellular and molecular changes associated with fish oocyte ageing. Rev. Aquacult. 1–12. DOI: 10.1111/raq.12249.
Samarin, A.M., Sampels, S., Krzyskow, A., Burkina, V., Kristan, J. et al. (2018b). Egg oxidation status, antioxidant enzyme activities, lipid classes, fatty acid composition profile and embryo survival rates during in vitro oocyte ageing in tench Tinca tinca (Linnaeus, 1758). Aquac. Res. 49(6): 2305–2316. DOI: 10.1111/are.13693.
Sasaki, H., Hamatani, T., Kamijo, S., Iwai, M., Kobanawa, M. et al. (2019). Impact of oxidative stress on age-associated decline in oocyte developmental competence. Front. Endocrinol. 10. DOI: 10.3389/fendo.2019.00811.
Somosy, Z. (2000). Radiation response of cell organelles. Micron 31: 165–181. DOI: 10.1016/S0968-4328(99)00083-9.
Sullivan, C.V., Chapman, R.W., Reading, B.J. & Anderson, P.E. (2015). Transcriptomics of mRNA and egg quality in farmed fish: some recent developments and future directions. General and comparative endocrinology 221: 23–30. DOI: 10.1016/j.ygcen.2015.02.012.
Tarín, J.J. Pérez-Albalá, S. & Cano, A. (2000). Consequences on offspring of abnormal function in ageing gametes. Hum. Reprod. Update 6(6): 532–549. DOI: 10.1093/humupd/6.6.532.
Yamamori, T., Yasui, H., Yamazumi, M., Wada, Y., Nakamura, Y. et al. (2012). Ionizing radiation induces mitochondrial reactive oxygen species production accompanied by upregulation of mitochondrial electron transport chain function and mitochondrial content under control of the cell cycle checkpoint. Free Radical Bio. Med. 53(2): 260–270. DOI: 10.1016/j.freeradbiomed.2012.04.033.
