Paper category: Original research paper
Corresponding author: Gonca Alak (galak@atauni.edu.tr)
DOI: https://doi.org/10.26881/oahs-2022.2.01
Received: 01/04/2022
Accepted: 20/04/2022
Full text: here
Citation (APA style): Alak,G.,Kotan,R.,Uçar,A.,Parlak,V. & Atamanalp,M.(2022).Pre-probiotic effects of different bacterial species in aquaculture: behavioral, hematological and oxidative stress responses. Oceanological and Hydrobiological Studies,51(2) 133-142. https://doi.org/10.26881/oandhs-2022.2.01
Abstract
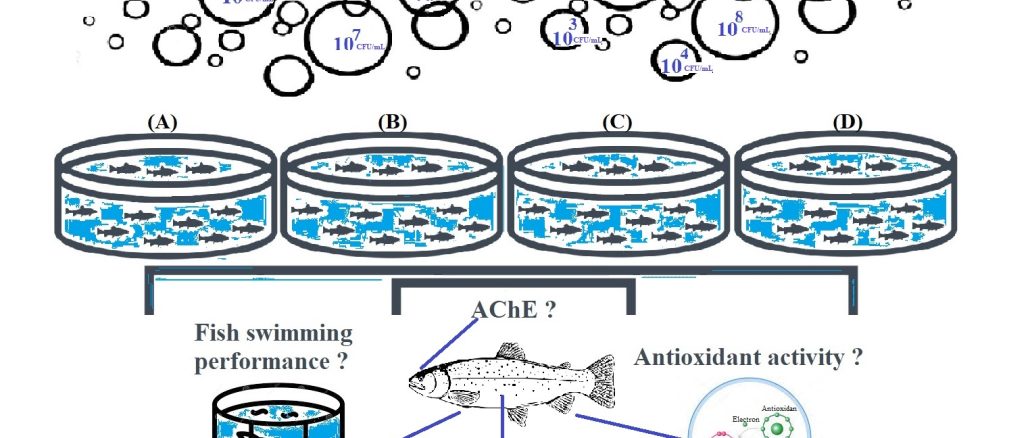
There is very limited aquaculture research on candidate probiotics and their effects on fish physiology. In this study, acute applications of four different molecularly identified bacterial species – Brevibacillus brevis FD-1 (A), Pseudomonas fluorescens FDG-37 (B), Bacillus sphaericus FD-48 (C), and B. amyloliquefaciens TV-17C (D), with potential in aquaculture, were tested in rainbow trout (Oncorhynchus mykiss) under static conditions. Physiological changes in blood tissue [hematological indices: erythrocyte count (RBC), leukocyte count (WBC), hemoglobin (Hb), hematocrit (Hct), platelet count (PLT), mean cell hemoglobin concentration (MCHC), mean cell hemoglobin (MCH), and mean cell volume (MCV)], oxidative stress responses in liver and gill tissues [malondialdehyde (MDA) level, antioxidant enzyme activities: superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), glutathione reductase (GR), glutathione-S-transferase (GST), glucose-6-phosphate dehydrogenase (G6PD)] and acetylcholinesterase (AChE) activity in brain tissue (as neurotoxic biomarker) were investigated. Additionally, behavioral differences were recorded by measuring swimming performance to support neurotoxic findings in all treatment groups. The LC5024 value of FDG-37 strain was determined through analysis as 1.0 × 108 CFU ml-1. Inhibition of enzyme activity, increase in the MDA level, as well as significant differences in hematological indices and swimming performance were determined in rainbow trout treated with B compared to control and other bacterial groups in gills. The potential for using group FD-48 and TV-17C bacterial strains as probiotics in aquaculture is more pertinent when considering the research findings and water quality parameters.
References
Aebi, H. (1974). Catalase. In: Methods of Enzymatic Analysis, Academic Press, New York, Bergmeyer, HU, USA, pp.673–678.
Alak, G., Özgeriş, F. B., Yeltekin, A. Ç., Parlak, V., Ucar, A., Caglar, O., Turkez, H., & Atamanalp, M. (2020b). Hematological and Hepatic Effects of Ulexite in Zebrafish. Environmental Toxicology and Pharmacology, 80, 103496. https://doi.org/10.1016/j.etap.2020.103496 PMID:32947019
Alak, G., Parlak, V., Ucar, A., Yeltekin, A. C., Ozgeris, F. B., Caglar, O., Atamanalp, M., & Turkez, H. (2020a). Oxidative and DNA damage potential of colemanite on zebrafish: Brain, liver and blood. Turkish Journal of Fisheries and Aquatic Sciences, 20(8), 593–602. https://doi.org/10.4194/1303-2712-v20_8_02
Alak, G., Yeltekin, A. Ç., Özgeriş, F. B., Parlak, V., Uçar, A., Sait Keleş, M., & Atamanalp, M. (2019). Therapeutic effect of N- acetyl cysteine as an antioxidant on rainbow trout’s brain in cypermethrin toxicity. Chemosphere, 221, 30–36. https://doi.org/10.1016/j.chemosphere.2018.12.196 PMID:30634146
Atamanalp, M., Parlak, V., Özgeriş, F. B., Çilingir Yeltekin, A., Ucar, A., Keleş, M. S., & Alak, G. (2021). Treatment of oxidative stress, apoptosis, and DNA injury with N-acetylcysteine at simulative pesticide toxicity in fish. Toxicology Mechanisms and Methods, 31(3), 224–234. https://doi.org/10.1080/15376516.2021.1871794 PMID:33412942
Beutler, E. (1984). Red Cell Metabolism: A Manual of Biochemical Methods (2nd ed.). Grune and Starton.
Beutler, E. (1984) Red Cell Metabolism: A Manual of Biochemical Methods, Second Edn., Grune and Starton, NewYork Bradford, M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 72, 248-254.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254. https://doi.org/10.1016/0003-2697(76)90527-3 PMID:942051
Chen, X., Yi, H., Liu, S., Zhang, Y., Su, Y., Liu, X., Bi, S., Lai, H., Zeng, Z., & Li, G. (2021). Probiotics Improve Eating Disorders in Mandarin Fish (Siniperca chuatsi) Induced by a Pellet Feed Diet via Stimulating Immunity and Regulating Gut Microbiota. Microorganisms, 9(6), 1288. https://doi.org/10.3390/microorganisms9061288 PMID:34204793
Dawood, M. A., Koshio, S., Abdel‐Daim, M. M., & Van Doan, H. (2019). Probiotic application for sustainable aquaculture. Reviews in Aquaculture, 11(3), 907–924. https://doi.org/10.1111/raq.12272
Harikrishnan, R., Balasundaram, C., Kim, M., Kim, J., & Heo, M. (2009). Effective administration route of azadirachtin and its impact on haematological and biochemical parameters in goldfish (Carassius auratus) infected with Aeromonas hydrophila. Bulletin of the Veterinary Institute in Pulawy, 53(4), 613–619.
Hassaan, M. S., El-Sayed, A. M. I., Mohammady, E. Y., Zaki, M. A., Elkhyat, M. M., Jarmołowicz, S., & El-Haroun, E. R. (2021). Eubiotic effect of a dietary potassium diformate (KDF) and probiotic (Lactobacillus acidophilus) on growth, hemato-biochemical indices, antioxidant status and intestinal functional topography of cultured Nile tilapia Oreochromis niloticus fed diet free fishmeal. Aquaculture (Amsterdam, Netherlands), 533, 736147. https://doi.org/10.1016/j.aquaculture.2020.736147
Hedayati, A., & Tarkhani, R. (2014). Hematological and gill histopathological changes in iridescent shark, Pangasius hypophthalmus (Sauvage, 1878) exposed to sublethal diazinon and deltamethrin concentrations. Fish Physiology and Biochemistry, 40(3), 715–720. https://doi.org/10.1007/s10695-013-9878-3 PMID:24126937
Jagtap, A. R., & Mali, R. P. (2012). Alterations in the erythrocyte sedimentation rate of fresh water fish, Channa punctatus on exposure to temperature stress from Godavari River, Nanded. International Journal of Biomedical and Advance Research, 3(12), 870–873.
Jahan, N., Islam, S. M., Rohani, M. F., Hossain, M. T., & Shahjahan, M. (2021). Probiotic yeast enhances growth performance of rohu (Labeo rohita) through upgrading hematology, and intestinal microbiota and morphology. Aquaculture (Amsterdam, Netherlands), 545, 737243. https://doi.org/10.1016/j.aquaculture.2021.737243
Kalaycı Kara, A., Fakıoğlu, Ö., Kotan, R., Atamanalp, M., & Alak, G. (2021). The investigation of bioremediation potential of Bacillus subtilis and B. thuringiensis isolates under controlled conditions in freshwater. Archives of Microbiology, 203, 2075–2085. https://doi.org/10.1007/s00203-021-02187-9 PMID:33595691
Köktürk, M., Yıldırım, S., Nas, M. S., Ozhan, G., Atamanalp, M., Bolat, I., Calimli, M. H., & Alak, G. (2021). Investigation of the oxidative stress response of a green synthesis nanoparticle (RP-Ag/ACNPs) in Zebrafish. Biological Trace Element Research, 1–11. https://doi.org/10.1007/s12011-021-02855-3 PMID:34403049
Kong, Y., Li, M., Chu, G., Liu, H., Shan, X., Wang, G., & Han, G. (2021). The positive effects of single or conjoint administration of lactic acid bacteria on Channa argus: Digestive enzyme activity, antioxidant capacity, intestinal microbiota and morphology. Aquaculture (Amsterdam, Netherlands), 531, 735852. https://doi.org/10.1016/j.aquaculture.2020.735852
Kuebutornye, F. K. A., Abarike, E. D., & Lu, Y. (2019). A review on the application of Bacillus as probiotics in aquaculture. Fish & Shellfish Immunology, 87, 820–828. https://doi.org/10.1016/j.fsi.2019.02.010 PMID:30779995
Pandiyan, P., Balaraman, D., Thirunavukkarasu, R., George, E. G. J., Subaramaniyan, K., Manikkam, S., & Sadayappan, B. (2013). Probiotics in aquaculture. Drug invention today, 5(1), 55-59. https://doi.org/10.1016/j.dit.2013.03.003
Saravanan, M., Kumar, K. P., & Ramesh, M. (2011). Haematological and biochemical responses of freshwater teleost fish Cyprinus carpio (Actinopterygii: Cypriniformes) during acute and chronic sublethal exposure to lindane. Pesticide Biochemistry and Physiology, 100(3), 206–211. https://doi.org/10.1016/j.pestbp.2011.04.002
Sun, Y., Oberley, L. W., & Li, Y. (1988). A simple method for clinical assay of superoxide dismutase. Clinical Chemistry, 34(3), 497–500. https://doi.org/10.1093/clinchem/34.3.497 PMID:3349599
Ucar, A., Özgeriş, F. B., Yeltekin, A. Ç., Parlak, V., Alak, G., Keleş, M. S., & Atamanalp, M. (2019). The effect of N-acetylcysteine supplementation on the oxidative stress levels, apoptosis, DNA damage, and hematopoietic effect in pesticide-exposed fish blood. Journal of Biochemical and Molecular Toxicology, 33(6), e22311. https://doi.org/10.1002/jbt.22311 PMID:30801904
Uçar, A., Parlak, V., Çilingir Yeltekin, A., Özgeriş, F. B., Çağlar, Ö., Türkez, H., Alak, G., & Atamanalp, M. (2021). Assesment of hematotoxic, oxidative and genotoxic damage potentials of fipronil in rainbow trout Oncorhynchus mykiss, Walbaum. Toxicology Mechanisms and Methods, 31(1), 73–80. https://doi.org/10.1080/15376516.2020.1831122 PMID:33050807
