Paper category: Original research paper
Corresponding author: Marek Klin (klin.m@outlook.com)
DOI: 10.1515/ohs-2018-0002
Received: 14 June 2017
Accepted: 01 August 2017
Full text: here
Citation (APA style):
Abstract
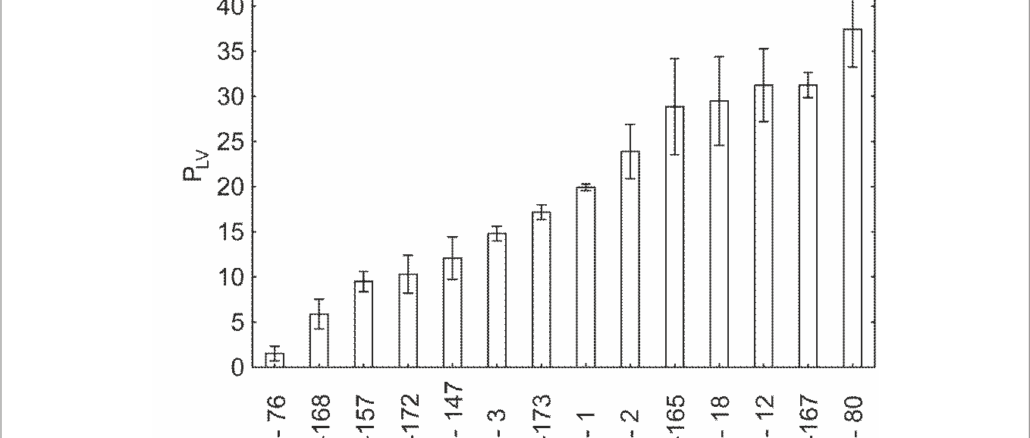
Screening of fourteen Baltic green algal strains provided basic data on their mass culture potential for the purpose of valuable biomass production with particular emphasis on lipid content. Selected microalgae were grown under non-stressed conditions in order to identify those characterized by efficient lipid production. The tested strains exhibited significant differences in growth patterns and lipid yields. Strains belonging to Chlorella and Stichococcus genera exhibited the highest growth rates, ranging from 0.39 d−1 to 0.50 d−1 and thus the highest final cell density (> 107 cells ml−1). Furthermore, five strains: C. minutissima BA-12, C. fusca BA-18, C. vulgaris BA-80, Monoraphidium sp. BA-165 and Chlorella sp. BA-167 were characterized by distinctively high lipid yield (> 60 mg l−1). The same strains, together with C. vulgaris BA-02, were also shown as those with the highest volumetric lipid productivity, reaching > 30 mg l−1 d−1. The tested Baltic strains performed well in terms of lipid production compared to the literature data, still leaving a great spectrum of opportunities for further lipid yield improvement.
References
Agusti, S., Duarte, C.M. & Kalff, J. (1987). Algal cell size and the maximum density and biomass of phytoplankton. Limnology and Oceanography 32(4): 983–986.
Banse, K. (1976). Rates of growth, respiration and photosynthesis of unicellular algae as related to cell size – a review. Journal of Phycology 12(2): 135–140. DOI: 10.1111/j.1529-8817.1976.tb00490.x
Bogen, C., Klassen, V., Wichmann, J., La Russa, M., Doebbe, A. et al. (2013). Identification of Monoraphidium contortum as a promising species for liquid biofuel production. Bioresource Technology 133: 622–626.
Borowitzka, M.A. (1992). Algal biotechnology products and processes – matching science and economics. Journal of Applied Phycology 4(3): 267–279. DOI: 10.1007/BF02161212.
Borowitzka, M.A. (2013). Species and Strain Selection. In M.A. Borowitzka & N.R. Mohaeimani (Eds.), Algae for Biofuels and Energy. (pp. 78–90). Dordrecht: Springer Science+Business Media.
Chabrol, E., & Charonnat, R. (1937). Une nouvelle reaction pour l’etude des lipides l’oleidemie. Presse Méd.
Courchesne, N.M.D., Parisien, A., Wang, B. & Lan, C.Q. (2009). Enhancement of lipid production using biochemical, genetic and transcription factor engineering approaches. Journal of Biotechnology 141(1–2): 31–41. DOI: 10.1016/j.jbiotec.2009.02.018.
Fogg, G.E (1975). Algal Cultures and Phytoplankton Ecology. 2nd edition, The University of Wisconsin Press, Wisconsin.
Folch, J., Lees, M., & Sloane Stanley, G. H. (1957). A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226(1): 497–509.
Grama, B.S., Chader, S., Khelifi, D., Stenuit, B., Jeffryes, C. et al (2014). Characterization of fatty acid and carotenoid production in an Acutodesmus microalga isolated from the Algerian Sahara. Biomass and Bioenergy 69: 265–275. DOI: 10.1016/j.biombioe.2014.07.023.
Griffiths, M.J., & Harrison, S.T.L. (2009). Lipid productivity as a key characteristic for choosing algal species for biodiesel production. Journal of Applied Phycology 21(5): 493–507. DOI: 10.1007/s10811-008-9392-7.
Griffiths, M.J., van Hille, R.P. & Harrison, S.T.L. (2012). Lipid productivity, settling potential and fatty acid profile of 11 microalgal species grown under nitrogen replete and limited conditions. Journal of Applied Phycology 24(5): 989–1001. DOI: 10.1007/s10811-011-9723-y.
Guillard, R.R.L. (1973). Division rates. In E. Stein, J.R. (Ed.), Handbook of Phycological Methods: Culture Methods and Growth Measurements (pp. 289–312). Cambridge: Cambridge University Press.
Guillard, R.R.L. (1975). Culture of Phytoplankton for Feeding Marine Invertebrates. In Culture of Marine Invertebrate Animals (pp. 29–60). Boston, MA: Springer US. DOI: 10.1007/978-1-4615-8714-9_3.
Harwati, T.U., Willke, T. & Vorlop, K.D. (2012). Characterization of the lipid accumulation in a tropical freshwater microalgae Chlorococcum sp. Bioresource Technology 121: 54–60. DOI: 10.1016/j.biortech.2012.06.098.
Hein, M., Pedersen, M.F. & Sand-Jensen, K. (1995). Size-dependent nitrogen uptake in micro- and macroalgae. Marine Ecology Progress Series 118: 247–253.
Illman, A.M., Scragg, A.H., & Shales, S.W. (2000). Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzyme and Microbial Technology 27(8): 631–635. DOI: 10.1016/S0141-0229(00)00266-0.
Khozin-Goldberg, I. (2016). Lipid Metabolism in Microalgae. In M.A. Borowitzka, J. Beardall & J.A. Raven (Eds.), The Physiology of Microalgae (pp. 413–481). Cham: Springer International Publishing. DOI: 10.1007/s00299-013-1493-3.
Knight, J.A., Anderson, S. & Rawle, J.M. (1972). Chemical Basis of the Sulfo-phospho-vanillin Reaction for Estimating Total Serum Lipids. Clinical Chemistry 18(3): 199–202.
Kong, W., Song, H., Cao, Y., Yang, H., Hua, S. et al. (2011). The characteristics of biomass production , lipid accumulation and chlorophyll biosynthesis of Chlorella vulgaris under mixotrophic cultivation. African Journal of Biotechnology 10(55): 11620–11630. DOI: 10.5897/AJB11.617.
Lam, M.K. & Lee, K.T. (2012). Microalgae biofuels: A critical review of issues, problems and the way forward. Biotechnology Advances 30(3): 673–690. DOI: 10.1016/j.biotechadv.2011.11.008.
Latała, A., Jodłowska, S. & Pniewski, F. (2006). Culture Collection of Baltic Algae (CCBA) and characteristic of some strains by factorial experiment approach. Algological Studies 122(December): 137–154. DOI: 10.1127/1864-1318/2006/0122-0137.
Lee, J.Y., Yoo, C., Jun, S.Y., Ahn, C.Y., & Oh, H.M. (2010). Comparison of several methods for effective lipid extraction from microalgae. Bioresource Technology 101(1 SUPPL.): S75–S77. DOI: 10.1016/j.biortech.2009.03.058.
Mata, T.M., Martins, A.A., Caetano, N.S., Nio, A., Martins, A.A. et al. (2010). Microalgae for biodiesel production and other applications: A review. Renewable and Sustainable Energy Reviews 14(1): 217–232. DOI: 10.1016/j.rser.2009.07.020.
Metzger, P. & Largeau, C. (2005). Botryococcus braunii: A rich source for hydrocarbons and related ether lipids. Applied Microbiology and Biotechnology 66(5): 486–496. DOI: 10.1007/s00253-004-1779-z.
Miner, B. G., Sultan, S. E., Morgan, S. G., Padilla, D. K., & Relyea, R. A. (2005). Ecological consequences of phenotypic plasticity. Trends in Ecology & Evolution 20(12): 685–692. DOI: 10.1016/j.tree.2005.08.002.
Nascimento, I.A., Marques, S.S.I., Cabanelas, I.T.D., Pereira, S.A., Druzian, J.I. et al. (2013). Screening Microalgae Strains for Biodiesel Production: Lipid Productivity and Estimation of Fuel Quality Based on Fatty Acids Profiles as Selective Criteria. Bioenergy Research 6(1): 1–13. DOI: 10.1007/s12155-012-9222-2.
Nielsen, S.L. (2006). Size-dependent growth rates in eukaryotic and prokaryotic algae exemplified by green algae and cyanobacteria: Comparisons between unicells and colonial growth forms. Journal of Plankton Research 28(5): 489–498. DOI: 10.1093/plankt/fbi134.
Olenina, I., Hajdu, S., Edler, L., Wasmund, N., Busch, S. et al. (2006). Biovolumes and size-classes of phytoplankton in the Baltic Sea. HELCOM Balt. Sea Environ. Proc. 106(106), 144 pp.
Olofsson, M., Lindehoff, E., Frick, B., Svensson, F. & Legrand, C. (2015). Baltic Sea microalgae transform cement flue gas into valuable biomass. Algal Research 11: 227–233. DOI: 10.1016/j.algal.2015.07.001.
Patterson, G.M.L., Larsen, L.K. & Moore, R.E. (1994). Bioactive natural products from blue-green algae. Journal of Applied Phycology 6: 151–157.
Schwenk, D., Seppälä, J., Spilling, K., Virkki, A., Tamminen, T. et al. (2013). Lipid content in 19 brackish and marine microalgae: influence of growth phase, salinity and temperature. Aquatic Ecology 47(4): 415–424. DOI: 10.1007/s10452-013-9454-z.
StatSoft, Inc. (2007). STATISTICA (data analysis software system), version 8.0. www.statsoft.com.
Stanisz, A. (2007a). Przystępny kurs statystyki z zastosowaniem STATISTICA PL na przykładach z medycyny. Tom 2. Modele liniowe i nieliniowe (pp. 271–314). Kraków: StatSoft Polska Sp.z.o.o. (In Polish).
Stanisz, A. (2007b). Przystępny kurs statystyki z zastosowaniem STATISTICA PL na przykładach z medycyny. Tom 3. Analizy wymiarowe (pp. 113–161). Kraków: StatSoft Polska Sp.z.o.o. (In Polish).
Spolaore, P., Joannis-Cassan, C., Duran, E. & Isambert, A. (2006). Commercial applications of microalgae. Journal of Bioscience and Bioengineering 101(2): 87–96. DOI: 10.1263/jbb.101.87.
Sydney, E.B., da Silva, T.E., Tokarski, A., Novak, A.C., de Carvalho, J.C. et al. (2011). Screening of microalgae with potential for biodiesel production and nutrient removal from treated domestic sewage. Applied Energy 88(10): 3291–3294. DOI: 10.1016/j.apenergy.2010.11.024.
Tan, K.W.M. & Lee, Y.K. (2016). The dilemma for lipid productivity in green microalgae: importance of substrate provision in improving oil yield without sacrificing growth. Biotechnology for Biofuels 9(1): 255. DOI: 10.1186/s13068-016-0671-2.
Tasi, M.B., Pinto, L.F.R., Klein, B.C., Veljkovi, V.B. & Filho, R.M. (2016). Botryococcus braunii for biodiesel production. Renewable and Sustainable Energy Reviews 64: 260–270. DOI: 10.1016/j.rser.2016.06.009.
Tsarenko, P., Borysova, O. & Blume, Y. (2016). High biomass producers and promising candidates for biodiesel production from microalgae collection IBASU-A(Ukraine). Oceanol. Hydrobiol. St. 45(1): 79–85. DOI: 10.1515/ohs-2016-0008.
Wood, M. A., Everroad, R. C., & Wingard, L. (2005). Measuring Growth Rates in Microalgal Cultures. In R.A. Andersen (Ed.), Algal Culturing Techniques (pp. 269–285). Burlington, USA. DOI: 10.1007/s13398-014-0173-7.2.
Wuang, S.C., Luo, Y.D., Wang, S., Chua, P.Q.D., & Tee, P.S. (2016). Performance assessment of biofuel production in an algae-based remediation system. Journal of Biotechnology 221: 43–48. DOI: 10.1016/j.jbiotec.2016.01.024.
Xu, Y. & Boeing, W.J. (2014). Modeling maximum lipid productivity of microalgae: Review and next step. Renewable and Sustainable Energy Reviews 32: 29–39. DOI: 10.1016/j.rser.2014.01.002.
Yoo, C., Jun, S.-Y., Lee, J.-Y., Ahn, C.-Y. & Oh, H.-M. (2010). Selection of microalgae for lipid production under high levels carbon dioxide. Bioresource Technology 101(1): S71-S74 DOI: 10.1016/j.biortech.2009.03.030.
Yu, X., Zhao, P., He, C., Li, J., Tang, X. et al. (2012). Isolation of a novel strain of Monoraphidium sp. and characterization of its potential application as biodiesel feedstock. Bioresource Technology 121: 256–262. DOI: 10.1016/j.biortech.2012.07.002.

Bądź pierwszy, który skomentuje ten wpis