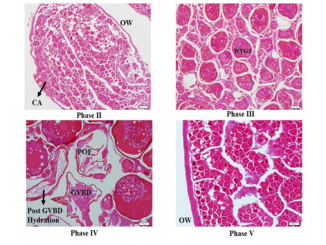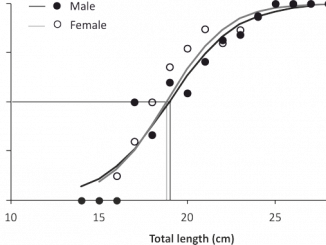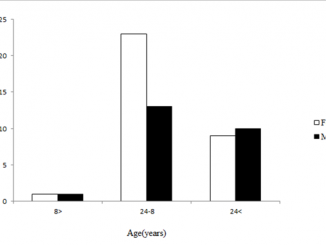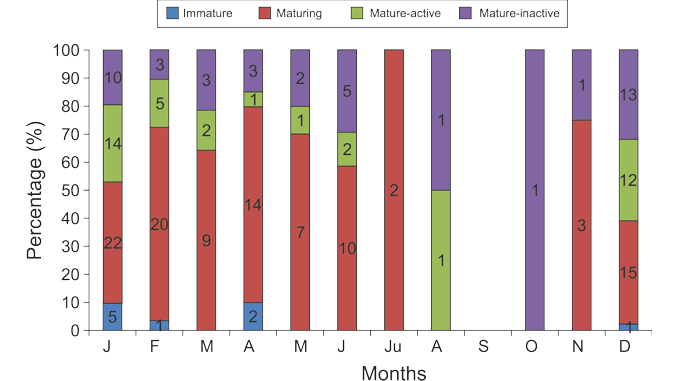
Paper category: Original research paper
Corresponding author: Richard Kindong (kindong_richard@yahoo.com)
DOI: https://doi.org/10.26881/oahs-2022.1.10
Received: 12/07/2021
Accepted: 08/11/2021
Full text: here
Citation (APA style): Kindong,R.,Wu,F.,Sarr,O.,Dai,L.,Tian,S. & Dai,X.(2022).Life history of wahoo, Acanthocybium solandri, in the Tropical Eastern Atlantic Ocean – the importance of applying a suite of methods for fisheries assessment in data-limited situations. Oceanological and Hydrobiological Studies,51(1) 115-132. https://doi.org/10.26881/oahs.2022.1.10
Abstract
Unassessed fisheries, mostly non-targeted fisheries, are now particularly predominant in many commercial fisheries and are critical to food security in developing countries. These fisheries typically lack reliable data essential for assessing their stocks, leaving them susceptible to overfishing and declining yield over time. This study proposes a framework for determining the life history and management of such fisheries. Data on the length composition and reproduction of wahoo Acanthocybium solandri, a common bycatch species in commercial fisheries, were obtained from observers aboard Chinese longline vessels in the Eastern Atlantic between 2010 and 2020 and were used as a case study. A comprehensive methodological approach was applied using data on this species to estimate its life history parameters, to evaluate biological reference points, and to provide proxies for the stock status. The final main growth parameters obtained were: L<sub>inf</sub> = 161.21 cm FL (157.34–194.68), K = 0.47/year (0.14–0.65); estimated size at first maturity was 89.6 cm FL. As assessed by the set of methods applied, the wahoo stock state was healthy in the Tropical Eastern Atlantic Ocean. This study advises against using a single approach to determining life history parameters in data-limited fisheries, as this may affect reference points and thus management recommendations. This study provides a route whereby many easy-to-apply methods can be used to understand the status of multiple stocks in poorly managed fisheries, and thus provide management plans.
References
Abobi, S. M., Mildenberger, T. K., Kolding, J., & Wolff, M. (2019). Assessing the exploitation status of main fisheries resources in Ghana’s reservoirs based on reconstructed catches and a length-based bootstrapping stock assessment method. Lake and Reservoir Management, 35(4), 415–434. Advance online publication. https://doi.org/10.1080/10402381.2019.1616340
Allain, V. 2003. Diet of mahi-mahi, wahoo and lancetfish in the western and central Pacific. In: 16th Meeting of the Standing Committee on Tuna and Billfish Working Paper, Secretariat of the Pacific Community, Noumea, p. 19.
Alverson, D. L., & Carney, M. J. 1975. A graphic review of the growth and decay of population cohorts. ICES Journal of Marine Science. 36(2), 133–143. https://doi.org/10.1093/icesjms/36.2.133
Beverton, R. J. H., & Holt, S. J. (1956). A review of the methods for estimating mortality rates in fish populations, with special reference to sources of bias in catch sampling. Rapports et Procès Verbaux des Réunions - Commission Internationale pour l’Exploration Scientifique de la Mer Méditerranée, 140, 67–83.
Beverton, R. J. H., & Holt, S. J. (1957). On the dynamics of exploited fish populations. Ministry of Agriculture.
Bjornda, L. K. A., Bolten, A. B., Coan, A. L., & Kleiber, P. (1995). Estimation of green turtle (Chelonia mydas) growth rates from length-frequency analysis. Copeia, 1995(1), 71–77. https://doi.org/10.2307/1446800
Brodziak, J., Ianelli, J., Lorenzen, K., & Methot, R. D., Jr. 2011. Estimating natural mortality in stock assessment applications. Department Commerce, NOAA, Tech Memo, NMFS-F/SPO-199, NMFS-F/SPO-199pg. (Washington: NOAA), 38.
Brown-Peterson, N. J., Franks, J. S., & Burke, A. M. (2000). Preliminary observations on the reproductive biology of wahoo, Acanthocybium solandri, from the northern Gulf of Mexico and Bimini, Bahamas. Proceedings of the Gulf and Caribbean Fisheries Institute, 51, 414–427.
Brown-Peterson, N. J., Wyanski, D. M., Saborido-Rey, F., Macewicz, B. J., & Lowerre-Barbieri, S. K. (2011). A Standardized Terminology for Describing Reproductive Development in Fishes. Marine and Coastal Fisheries, 3(1), 52–70. https://doi.org/10.1080/19425120.2011.555724
Campana, S. E. (2001). Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. Journal of Fish Biology, 59(2), 197–242. https://doi.org/10.1111/j.1095-8649.2001.tb00127.x
Carbonara, P., Intini, S., Kolitari, J., Joksimović, A., Milone, N., Lembo, G., Casciaro, L., Bitetto, I., Zupa, W., Spedicato, M. T., & Sion, L. (2018). A holistic approach to the age validation of Mullus barbatus L., 1758 in the Southern Adriatic Sea (Central Mediterranean). Scientific Reports, 8, 13219. https://doi.org/10.1038/s41598-018-30872-1 PMID:30185811
Chen, S., & Watanabe, S. (1989). Age Dependence of Natural Mortality Coefficient in Fish Population Dynamics. Nippon Suisan Gakkaishi, 55(2), 205–208. https://doi.org/10.2331/suisan.55.205
Collette, B. B., & Nauen, C. E. 1983. FAO Species Catalogue Vol. 2 Scombrids of the world. FAO Fisheries Synopsis, 125, Rome. 137 pp.
Costello, C., Ovando, D., Hilborn, R., Gaines, S. D., Deschenes, O., & Lester, S. E. (2012). Status and solutions for the world’s unassessed fisheries. Science, 338(6106), 517–520. https://doi.org/10.1126/science.1223389 PMID:23019613
Figuerola-Fernandez, M., Pena-Alvarado, N., & Torres-Ruiz, W. (2008). 2008. Maturation and reproductive seasonality of the wahoo (Acanthocybium solandri), red-ear sardine (Harengula humeralis), false pilchard (Harengula clupeola), thread herring (Opisthonema oglinum), crevalle jack (Caranx hippos), horse-eye jack (Caranx latus), blue runner (Caranx crysos), and great barracuda (Sphyraena barracuda) in Puerto Rico. In J. Velez-Arocho, E. Diaz-Velazquez, M. Garcia-Perez, J. M. Berrios, & A. Rosario-Jimenez (Eds.), Aspects of the reproductive biology of recreationally important fish species in Puerto Rico. Department of Natural and Environmental Resources, Fish and Wildlife Bureau.
Froese, R., Demirel, N., Coro, G., Kleisner, K. M., & Winker, H. (2017). Estimating fisheries reference points from catch and resilience. Fish and Fisheries, 18(3), 506–526. https://doi.org/10.1111/faf.12190
Froese, R., Winker, H., Coro, G., Demirel, N., Tsikliras, A. C., Dimarchopoulou, D., Scarcella, G., Probst, W. N., Dureuil, M., & Pauly, D. (2018). A new approach for estimating stock status from length frequency data. ICES Journal of Marine Science, 75(6), 2004–2015. https://doi.org/10.1093/icesjms/fsy078
Gao, C., Tian, S., Kindong, R., & Dai, X. (2020). Biology and Environmental Preferences of Wahoo, Acanthocybium solandri (Cuvier, 1832), in the Western and Central Pacific Ocean (WCPO). Journal of Marine Science and Engineering, 8(3), 184. https://doi.org/10.3390/jmse8030184
Gayanilo, F. C., & Pauly, D. (Eds.). (1997). “FAO-ICLARM Stock Assessment Tools: Reference Manual,” in FAO Computerized Information Series (Fisheries) 8. Food and Agriculture Organization of the United Nations.
Gayanilo, F. C., Sparre, P., & Pauly, D. (2005). FAO-ICLARM stock assessment tools II: User’s guide. Food & Agriculture Organization.
Gislason, H., Daan, N., Rice, J. C., & Pope, J. G. (2010). Size, growth, temperature and the natural mortality of marine fish. Fish and Fisheries, 11(2), 149–158. https://doi.org/10.1111/j.1467-2979.2009.00350.x
González-Gómeza, R., Meiners-Mandujanob, C., Morillo-Velardeb, P. S., Jiménez-Badillob, L., & Markaida, U. (2020). Reproductive dynamics and population structure of octopus insularis from the veracruz reef system marine protected area, Mexico. Fisheries Research, 221, 105385. https://doi.org/10.1016/j.fishres.2019.105385
Gulland, J.A. 1971. The fish resources of the ocean. West Byfleet (UK): Fishing News (Books) Ltd
Herrón, P., Mildenberger, T. K., Diaz, J. M., & Wolff, M. (2018). Assessment of the stock status of small-scale and multi-gear fisheries resources in the tropical Eastern Pacific region. Regional Studies in Marine Science, 24, 311–323. Advance online publication. https://doi.org/10.1016/j.rsma.2018.09.008
Hogarth, W. T. 1976. Life-history aspects of the wahoo Acanthocybium solandri (Cuvier and Valenciennes) from the coast of North Carolina. PhD Dissertation. North Carolina State University, Raleigh, NC, USA.
Haddon, M. (2011). Modelling and Quantitative Methods in Fisheries (2nd ed.). CRC Press. https://doi.org/10.1201/9781439894170
Hewitt, D. A., & Hoenig, J. M. (2005). Comparison of two approaches for estimating natural mortality based on longevity. Fish Bulletin, 103, 433–437.
Hoenig, J. M. (1983). Empirical Use of Longevity Data to Estimate Mortality Rates. Fish Bulletin, 82, 898–903.
Hilborn, R., & Walters, C. J. (1992). Quantitative fisheries stock assessment: choice, dynamics and uncertainty. Chapman and Hall. https://doi.org/10.1007/978-1-4615-3598-0
Hordyk, A., Ono, K., Valencia, S., Loneragan, N., & Prince, J. (2015). A novel length-based empirical estimation method of spawning potential ratio (SPR), and tests of its performance, for small-scale, data-poor fisheries. ICES Journal of Marine Science, 72(1), 217–231. https://doi.org/10.1093/icesjms/fsu004
ICCAT (INTERNATIONAL COMMISSION for the CONSERVATION of ATLANTIC TUNAS). (2019). Report for Biennial Period, 2018-19, Part I, Vol. 2, 194, 252, 351pp. Iversen, E.S., & Yoshida, H.O.1957. Notes on the biology of the wahoo in the Line Islands. Pacific Science, 11, 370–379.
Jenkins, K. L. M., & McBride, R. S. (2009). Reproductive biology of wahoo, Acanthocybium solandri, from the Atlantic coast of Florida and the Bahamas. Marine and Freshwater Research, 60(9), 893–897. https://doi.org/10.1071/MF08211
Jons, G. D., & Miranda, L. E. (1997). Ovarian weight as an index of fecundity, maturity, and spawning periodicity. Journal of Fish Biology, 50(1), 150–156. https://doi.org/10.1111/j.1095-8649.1997.tb01347.x
Kenchington, T. J. (2014). Natural mortality estimators for information-limited fisheries. Fish and Fisheries, 15(4), 533–562. https://doi.org/10.1111/faf.12027
Kindong, R., Zhu, J. F., Dai, X. J., & Tian, S. Q. (2019). Life history parameters and yield per recruit analysis for Tachysurus nitidus and Plagiognathops microlepis in lake Dianshan and their management implications. Turkish Journal of Fisheries and Aquatic Sciences, 19(12). Advance online publication. https://doi.org/10.4194/1303-2712-v19_12_05
Kindong, R., Gao, C., & Tian, S. (2020). Achille Pandong, Qiuyun Ma, Feng Wu & Ousmane Sarr. 2020. Stock status assessments of five small pelagic species in the Atlantic and Pacific Oceans using the Length-Based Bayesian Estimation (LBB) Method. Frontiers in Marine Science, 7, 592082. Advance online publication. https://doi.org/10.3389/fmars.2020.592082
Kirkwood, G. P., & Zara, S. J. (2001). Length Frequency Distribution Analysis (LFDA), Version 5.0. MRAG Ltd.
Kishore, R., & Chin, X. 2001. Age and growth studies at the CFRAMP/IMA regional age and growth laboratory progress of work done and future approaches. In: SinghRenton, S. (Ed.), CARICOM Fishery Report 9, CARICOM Fisheries Unit Belize City, Belize, pp. 74–89
Lee, T. M. 2008. Estimation of life history parameters, biological reference points, and associated uncertainties for wahoo (Acanthocybium solandri) in the waters off eastern Taiwan. Master’s thesis, National Taiwan University.
Lorenzen, K. (2000). Allometry of natural mortality as a basis for assessing optimal release size in fish-stocking programmes. Canadian Journal of Fisheries and Aquatic Sciences, 57(12), 2374–2381. https://doi.org/10.1139/f00-215
Luan. S.H., Dai, X.J., Tian, S.Q., Li, W.W., and Fan, Y.C. 2017. Biology and environmental preferences of Acanthocybium solandri in the southeast Pacific Ocean. Marine Fisheries, Vol: 1-0030-11 (In Chinese).
Mazerolle, Marc J. 2019. Package ‘AICcmodavg’: Model Selection and Multi-model Inference Based on (Q)AIC(c); Version 2.2-2.
McBride, R. S., Richardson, A. K., & Maki, K. L. (2008). Age, growth, and mortality of wahoo, Acanthocybium solandri, from the Atlantic coast of Florida and the Bahamas. Marine and Freshwater Research, 59(9), 799–807. https://doi.org/10.1071/MF08021
Mildenberger, T. K., Taylor, M. H., & Wolff, M. (2017). TropFishR: An R package for fisheries analysis with length-frequency data. Methods in Ecology and Evolution, 8(11), 1520–1527. https://doi.org/10.1111/2041-210X.12791
Mildenberger, T. K. 2017. Single-species fish stock assessment with TropFishR. https://cran r-project org/web/packages/TropFishR/vignettes/tutorial html
Murray, P. A., & Sarvay, W. B. (1987). Use of ELEFAN programs in the estimation of growth parameters of the wahoo, Acanthocybium solandri, caught off St. Lucia, West Indies. Fishbyte, 5, 14–15.
Murray, P. A., & Joseph, W. B. (1996). Trends in the exploitation of the wahoo, Acanthocybium solandri, landed in St. Lucia. Proceedings of the Gulf and Caribbean Fisheries Institute, 44, 737–746.
Oxenford, H. A., Murray, P. A., & Luckhurst, B. E. (2003). The biology of wahoo (Acanthocybium solandri) in the western central Atlantic. Gulf and Caribbean Research, 15(1), 33–49. https://doi.org/10.18785/gcr.1501.06
Pantazi, V., Mannini, A., Vasilakopoulos, P., Kapiris, K., Megalofonou, P., & Kalogirou, S. (2020). That’s All I Know: Inferring the Status of Extremely Data-Limited Stocks. Frontiers in Marine Science, 7, 583148. https://doi.org/10.3389/fmars.2020.583148
Pauly, D., & Munro, J. (1984). Once more on the comparison of growth in fish and invertebrates. Fishbyte, 2(1), 4–21.
Pauly, D. (1980). On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. ICES Journal of Marine Science, 39(2), 175–192. https://doi.org/10.1093/icesjms/39.2.175
Pauly, D., & David, N. (1981). ELEFAN I, a basic program for the objective extraction of growth parameters from length-frequency
Pons, M., Kell, L., Rudd, M. B., Cope, J. M., & Lucena-Fredou, F. (2019a). Performance of length-based data-limited methods in a multifleet context: Application to small tunas, mackerels, and bonitos in the Atlantic Ocean. ICES Journal of Marine Science, 76(4), 960–973. https://doi.org/10.1093/icesjms/fsz004
Pons M., Lucena-Fredou F., Fredou T., Mourato B. 2019b. Implementation of length-based and catch-based data limited methods for small tunas. SCRS/2019/063.
Pons, M., Cope, J. M., & Kell, L. T. (2020). Comparing performance of catch-based and length-based stock assessment methods in data-limited fisheries. Canadian Journal of Fisheries and Aquatic Sciences, 77(6), 1026–1037. https://doi.org/10.1139/cjfas-2019-0276
Quinn, T. J., & Deriso, R. B. (1999). Quantitative fish dynamics. Oxford University Press, Inc.
Rudd, M. B., & Thorson, J. T. (2018). Accounting for variable recruitment and fishing mortality in length-based stock assessments for data-limited fisheries. Canadian Journal of Fisheries and Aquatic Sciences, 75(7), 1019–1035. https://doi.org/10.1139/cjfas-2017-0143
R Development Core Team. 2020. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org
Shepherd, J. G. 1987. A weakly parametric method for estimating growth parameters from length composition data, p. 113-119. In D. Pauly and G.R. Morgan (eds). Length-based methods in fisheries research. ICLARM Conf. Proc. 13.
Sparre, P., & Venema, S. C. (1998). Introduction to Tropical Fish Stock Assessment. Part 1. Manual. FAO Fisheries Technical Paper. No. 306.1, Rev. 2. FAO.
Schwamborn, R., Mildenberger, T. K., & Taylor, M. H. 2018. R package version 0.1. Fishboot: Bootstrap-based Methods for the Study of Fish Stocks and Aquatic Populations. https://github.com/rschwamborn/fishboot
Schwamborn, R., Mildenberger, T. K., & Taylor, M. H. 2019.Assessing source of uncertainty in length-based estimates of body growth in populations of fishes and macroinvertebrates with bootstrapped ELEFAN. Journal of Ecological Modeling. https://doi.org/10.1016/j.ecolmodel.2019.01.003
Scrucca, L. (2013). GA: A package for genetic algorithms in R. Journal of Statistical Software, 53(4), 1–37. https://doi.org/10.18637/jss.v053.i04
Taylor, M. H. 2017. fishdynr: fisheries science related
population dynamics models. https://github.com/marchtaylor/fishdynr
Taylor, M. H., & Mildenberger, T. K. 2017. Extending electronic length frequency analysis in R. Fisheries Management and Ecology. 24, 330–338. 12232. https://doi.org/10.1111/fme.12232
Theisen, T. C., Bowen, B. W., Lanier, W., & Baldwin, J. D. (2008). High connectivity on a global scale in the pelagic wahoo, Acanthocybium solandri (tuna family Scombridae). Molecular Ecology, 17(19), 4233–4247. https://doi.org/10.1111/j.1365-294X.2008.03913.x PMID:19378403
Then, A. Y., Hoenig, J. M., Hall, N. G., Hewitt, D. A., & Jardim, H. E. E. (2015). Evaluating the predictive performance of empirical estimators of natural mortality rate using information on over 200 fish species. ICES Journal of Marine Science, 72, 82–92. https://doi.org/10.1093/icesjms/fsu136
Viana, D., Hazin, F. H. V., Nunes, D. M., Carvalho, F. C., Véras, D. P., & Travassos, P. (2008). Wahoo Acanthocybium solandri fishery in the vicinity of Saint Peter and Saint Paul Archipelago, Brazil, from 1998 to 2006. Collect. Vol. Sci. Pap. ICCAT, 62, 1662–1670.
Viana, D., Branco, I., Fernandes, C., Fischer, A., Carvalho, F., Travassos, P., & Hazin, F. 2013. Reproductive biology of the wahoo, Acanthocybium solandri (Teleostei: Scombridae) in the Saint Peter and Saint Paul Archipelago, Brazil. International Journal of Plant and Animal Science. ISSN: 2167-0437 Vol. 1 (4), pp. 049-057.
von Bertalanffy, L. (1983). A quantitative theory of organic growth (inquiries on growth laws II). Human Biology, 10, 181–213.
Xiang, Y., Gubian, S., Suomela, B., & Hoeng, J. (2013). Generalized simulated annealing for global optimization: The GenSA Package. The R Journal, 5(1), 13–28. http://rjournal.github.io/archive/2013-1/xiang-gubian-suomela-etal.pdf https://doi.org/10.32614/RJ-2013-002
Zischke, M. T., Griffiths, S. P., & Tibbetts, I. R. (2013a). Rapid growth of wahoo (Acanthcybium solandri) in the Coral Sea, based on length-at-age estimates using annual and daily increments on sagittal otoliths. ICES Journal of Marine Science, 70(6), 1128–1139. https://doi.org/10.1093/icesjms/fst039
Zischke, M. T., Farley, J. H., Griffiths, S. P., & Tibbetts, I. R. (2013b). Reproductive biology of wahoo, Acanthocybium solandri, off eastern Australia. Reviews in Fish Biology and Fisheries, 23, 491–506. Advance online publication. https://doi.org/10.1007/s11160-013-9304-z
Zischke, M. T., & Griffiths, S. P. (2015). Per-recruit stock assessment of wahoo (Acanthocybium solandri) in the southwest Pacific Ocean. Fishery Bulletin (Washington, D.C.), 113(4), 407–418. Advance online publication. https://doi.org/10.7755/FB.113.4.4