Paper category: Original research paper
Corresponding author: Ines Kovačić (ikovacic@unipu.hr)
DOI: 10.1515/ohs-2017-0041
Received: February 14, 2017
Accepted: May 23, 2017
Full text: here
Citation (APA style):
Abstract
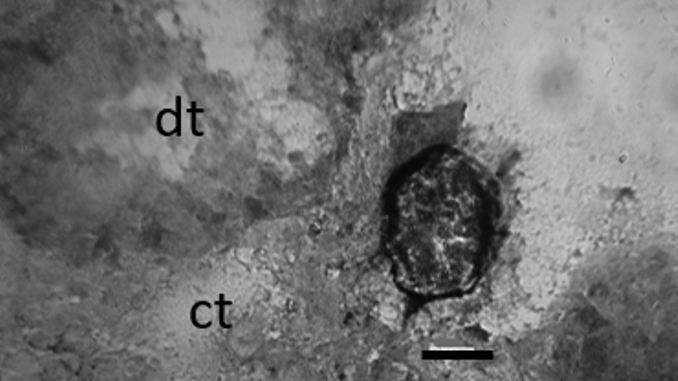
Histology has been used in the past to investigate the effects of diseases and parasite infections in native mussel populations that are often used as sentinel species in coastal environmental monitoring and as stock in mariculture. This paper presents the first study of parasite diversity using cryosections of the Mytilus galloprovincialis Lamarck, 1819 digestive gland. Mussels were sampled across the annual cycle at two sampling sites: St. Andrew and ACI Marina in the Northern Adriatic (Croatia). The protozoan of the genus Nematopsis Schneider, 1892 (Apicomplexa, Gregarina) was detected in digestive tubules, while the turbellarian (Urastomidae) Urastoma cyprinae von Graff, 1913 was found in the connective tissue at the edge of the digestive gland. The filamentous fungus Alternaria sp. (Fungi, Ascomycota) was detected in epithelial cells of the digestive tubule in cryosections. Nematopsis sp. occurred with the prevalence ranging from 20 to 100%, and the intensity of infection in less than 30 oocysts in most of the cases. U. cyprinae was detected in mussels sampled at St. Andrew and had a prevalence of 20% in September. Conidia of Alternaria sp. were found in mussels sampled at St. Andrew in September and November. Cryosections provide a useful and affordable means for monitoring parasites and endobiotic fungi.
References
Bayne, B.L. (1976). Marine Mussels, Their Ecology and Physiology. Cambridge University Press.
Bihari, N., Mičić, M., Fafanđel, M., Hamer, B., Jakšić et al. (2004). Seawater quality of Adriatic coast, Croatia, based on toxicity, genotoxicity and DNA integrity assay. Acta Adriatica 45(1): 75-81.
Boehs, G., Villalba, A., Ceuta, L.O. & Luz, J.R. (2010). Parasites of three commercially exploited bivalve mollusc species of the estuarine region of the Cachoeira river (Ilheus, Bahia, Brazil). Journal of Invertebrate Pathology 103(1): DOI: 43-47.10.1016/j.jip.2009.10.008.
Borzykh, O.G. & Zvereva, L.V. (2010). Filamentous fungi associated with the giant oyster Сrassostrea gigas (Bivalvia) from Peter the Great Bay of the Sea of Japan. Marine Ecosystems under the Global Change in the Northwestern Pacific. Proceedings of the China-Russia Bilateral Symposium on “Comparison on Marine Biodiversity in the Northwest Pacific Ocean”, 10-11 October 2010 (pp. 71-74). Qingdao, China: Institute of Oceanology, Chinese Academy of Sciences.
Borzykh, O.G. & Zvereva, L.V. (2015). Mycobiota of the bivalve mollusk Anadara broughtoni (Schrenck, 1867) from various parts of Peter the Great Bay, Sea of Japan. Russian Journal of Marine Biology 41(4): 321-323. DOI: 10.1134/S1063074015040033.
Bratoš, A., Glamuzina, B. & Benović, A. (2004). Hrvatsko školjkarstvo – prednosti i ograničenja (Croatian shellfisheries aquaculture – advantages and disadvantages). Naše more 51(1-2): 59-62.
Brenner, M. (2010). Assessing the health of blue mussels (Mytilus edulis) for site-selection of cultivation areas: potentials and constraints of applied parameters., ICES – Annual Science Conference, 23. September 2010, Nantes, France: International Council for the Exploration of the Sea.
Brenner, M., Buchholz, C., Heemken, O., Buck, B.H. & Koehler, A. (2012). Health and growth performance of the blue mussel (Mytilus edulis L.) from two hanging cultivation sites in the German Bight: a nearshore-offshore comparison. Aquaculture International 20(4): 751-778. DOI: 10.1007/s10499-012-9501-0.
Bush, A.O., Lafferty, K.D., Lotz, J.M. & Shostak, A.W. (1997). Parasitology meets ecology on its own terms: Margolis et al. revisited. Journal of Parasitology 83(4): 575-583.
Caceres-Martinez, J., Vasquez-Yeomans, R. & Sluys, R. (1998). The turbellarian Urastoma cyprinae from edible mussels Mytilus galloprovincialis and Mytilus californianus in Baja California, NW Mexico. Journal of Invertebrate Pathology 72(3): 214-219.
Canestri-Trotti, G., Baccarani, E.M., Paesanti, F. & Turolla, E. (2000). Monitoring of infections by protozoa of the genera Nematopsis, Perkinsus and Porospora in the smooth venus clam Callista chione from the North-Western Adriatic Sea (Italy). Diseases of Aquatic Organisms 42(2): 157-161. DOI: 10.3354/dao042157.
Ceuta, L.O. & Boehs, G. (2012). Parasites of the mangrove mussel Mytella guyanensis (Bivalvia: Mytilidae) in Camamu Bay, Bahia, Brazil. Brazilian Journal 72(3): 421-427. DOI: 10.1590/S1519-69842012000300002.
Cova, A.W., Serafim Júnior, M., Boehs, G. & Souza, J.M.D. (2015). Parasites in the mangrove oyster Crassostrea rhizophorae cultivated in the estuary of the Graciosa River in Taperoá, Bahia. Revista Brasileira de Parasitologia Veterinária 24(1): 21-27. DOI: 10.1590/S1984-29612015012.
Crespo-González, C., Rodríguez-Domínguez, H., Segade, P., Iglesias, R., Arias, C. et al. (2010). Seasonal Dynamics and Microhabitat Distribution of Urastoma cyprinae in Mytilus galloprovincialis: Implications for Its Life Cycle. Journal of Shellfish Research 29(1): 187-192. DOI: 10.2983/035.029.0113.
Darriba, S., Iglesias, D., Ruiz, M., Rodriguez, R. & Lopez, C. (2010). Histological survey of symbionts and other conditions in razor clam Ensis arcuatus (Jeffreys, 1865) (Pharidae) of the coast of Galicia (NW Spain). Journal of Invertebrate Pathology 104(1): 23-30. DOI: 10.1016/j.jip.2009.12.005.
FAO. (2015). National Aquaculture Sector Overview. Rome, Italy. (www.fao.org).
Final report. (2014). Coastal Cities Pollution project 2, The Adriatic Sea Monitoring Program 2nd Phase. Center for Marine Research Split. 8-9.
Francisco, C.J., Hermida, M.A. & Santos, M.J. (2010). Parasites and symbionts from Mytilus galloprovincialis (Lamark, 1819) (Bivalves: Mytilidae) of the Aveiro estuary Portugal. Journal of Parasitology 96(1): 200-205. DOI: 10.1645/GE-2064.1.
Gaevskaya, А.В. (2006). Parasite, diseases and pests of mussels (Mytilus, Mytilidae). II. Mollusca. I. Protozoa. Sevastopol.
Hamer, B., Korlević, M., Durmiši, E., Nerlović, V. & Bierne, N. (2012). Nuclear marker Me 15-16 analyses of Mytilus galloprovincialis populations along the eastern Adriatic coast. Cahiers de Biologie Marine 53(1): 35-44.
Jiménez, R., de Barniol, L. & Machuca, M. (2002). Nematopsis marinus n. sp., a new septate gregarine from cultured penaeoid shrimp Litopenaeus vannamei (Boone), in Ecuador. Aquaculture Resesearch 33(4): 231-240. DOI: 10.1046/j.1355-557x.2002.00647.x.
Kovačić, I., Fafanđel, M. & Bihari, N. (2015). Lysosomal deoxyribonuclease II from the mussel Mytilus galloprovincialis: Characterization and seasonal activity. Marine Biology Research 11(7): 716-724. DOI: 10.1080/17451000.2014.985232.
Kovačić, I., Pavičić-Hamer, D., Pfannkuchen, M. & Usich, M. (2016). Mytilus galloprovincialis (Lamarck, 1819) as host of Mytilicola orientalis (Mori, 1935) in the northern Adriatic Sea: presence and effect. Aquaculture International 25(1): 211-221. DOI: 10.1007/s10499-016-0023-z.
Marušić, N., Vidaček, S., Medić, H. & Petrak, T. (2009). Index kondicije dagnji (Mytilus galloprovincialis) u uvali Budava i zaljevu Raša. Ribarstvo 67(3): 19-25.
Mladineo, I. (2008). Risk assessment of parasitic/symbiotic organisms of the commercially important mytilid Modiolus barbatus (Linnaeus, 1758). Aquaculture Research 39(16): 1705-1719. DOI: 10.1111/j.1365-2109.2008.02047.x.
Mladineo, I., Petrić, M., Hrabar, J., Bočina, I. & Peharda, M. (2012). Reaction of the mussel Mytilus galloprovincialis (Bivalvia) to Eugymnanthea inquilina (Cnidaria) and Urastoma cyprinae (Turbellaria) concurrent infestation. Journal of Invertebrate Pathology 110(1): 118-125. DOI: 10.1016/j.jip.2012.03.001.
Özer, A. & Güneydağ, S. (2015a). First report of some parasites from Mediterranean mussel, Mytilus galloprovincialis Lamarck, 1819, collected from the Black Sea coast at Sinop. Turkish Journal of Zoology 38: 486-490. DOI:10.3906/zoo-1401-2.
Özer, A. & Güneydağ, S. (2015b). Seasonality and host-parasite interrelationship of Mytilus galloprovincialis parasites in Turkish Black Sea coasts. Journal of the Marine Biological Association of the United Kingdom 95(8): 1591-1599. DOI: 10.1017/S0025315415000740.
Pavičić-Hamer, D., Kovačić, I., Koščica, L. & Hamer, B. (2016). Physiological Indices of Maricultured Mussel Mytilus galloprovincialis Lamarck, 1819 in Istria, Croatia: Seasonal and Transplantation Effect. Journal of the World Aquaculture Society 47(6): 768-778. DOI: 10.1111/jwas.12316.
Rayyan, A., Photis, G. & Chintiroglou, C.C. (2004). Metazoan parasite species in cultured mussel Mytilus galloprovincialis in the Thermaikos Gulf (North Aegean Sea, Greece). Diseases of Aquatic Organisms 58(1): 55-62. DOI: 10.3354/dao058055.
Saffo, M.B. (1992). Coming to terms with a field: words and concepts in symbiosis. Symbiosis 14(1): 17-31.
Tuntiwaranuruk, C., Chalermwat, K., Upatham, E.S., Kruatrachue, M. & Azevedo, C. (2004). Investigation of Nematopsis spp. oocysts in 7 species of bivalves from Chonburi province, Gulf of Thailand. Diseases of Aquatic Organisms 58(1): 47-53. DOI: 10.3354/dao058047.
Tuntiwaranuruk, C., Chalermwat, K., Pongsakchat, V., Meepool, A., Upatham, E.S. et al. (2008). Infection of Nematopsis oocysts in different size classes of the farmed mussel Perna viridis in Thailand. Aquaculture 281(1-4): 12-16. DOI: 10.1016/j.aquaculture.2008.05.025.
Venugopal, V. (2008). Marine products for healthcare: functional and bioactive nutraceutical compounds from the ocean. CRC press, 371-393.
Zhang, Y., Mu, J., Feng, Y., Kang, Y., Zhang, J. et al. (2009). Broad-Spectrum Antimicrobial Epiphytic and Endophytic Fungi from Marine Organisms: Isolation, Bioassay and Taxonomy. Marine Drugs 7(2): 97-112. DOI: 10.3390/md7020097.
Zvereva, L.V. & Borzykh, O.G. (2010). Fungal complexes associated with the Primorsky scallop Mizuhopecten yessoensis and the mussel Mytilus trossulus (Bivalvia) from polluted and clean areas of the Peter the Great Bay, Sea of Japan, Proceedings of China-Russia Bilatteral symposium on “Comparison on marine biodiversity in the northwest Pacific ocean” 10-11 October 2010 (pp. 220-224). Qingdao, China: Institute of Oceanology, Chinese Academy of Sciences.
Zvereva, L.V. & Vysotskaya, M.A. (2005). Filamentous fungi associated with bivalve mollusks from polluted biotopes of Ussuriiskii Bay, Sea of Japan. Russian Journal of Marine Biology 31(6): 382-385. DOI: 10.1007/s11179-006-0007-3.

Bądź pierwszy, który skomentuje ten wpis