Paper category: Original research paper
Corresponding author: Mohsen M. El-Sherbiny (ooomar@kau.edu.sa)
DOI: 10.2478/oandhs-2021-0010
Received: 15/08/2020
Accepted: 22/10/2020
Full text: here
Citation (APA style): El-Sherbiny, M., Al-Harbi, M. & Kumar, A. (2021). Spatio-temporal variation of microphytoplankton communities in Obhur Creek, the central Red Sea. Oceanological and Hydrobiological Studies, 50(1), 98-114. https://doi.org/10.2478/oandhs-2021-0010
Abstract
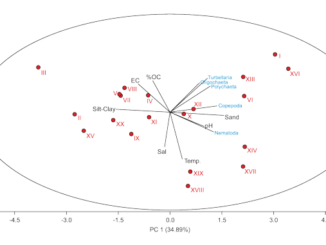
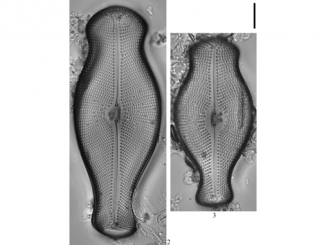
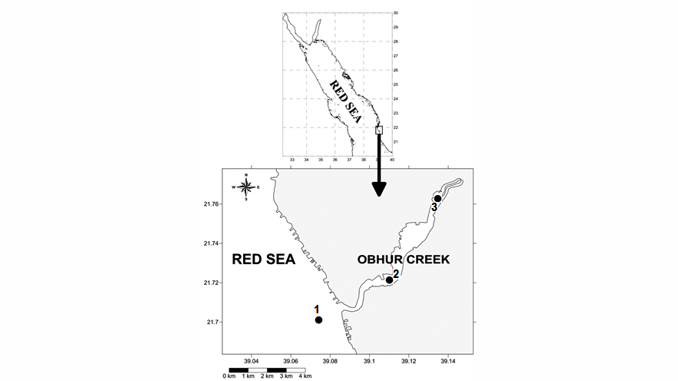
The abundance and distribution of microphyto-plankton and related physicochemical factors were assessed monthly in Obhur Creek, the central Red Sea. Sampling was carried out near the entrance, the middle and end parts of the creek. During the course of the present study, the Red Sea was characterized by predominantly oligotrophic conditions. Nutrient concentrations were relatively higher in the end part of the creek compared to the two other study sites. Chlorophyll a was also low throughout the year (average: 0.35 ± 0.32 mg m−3), except in May when it showed clear peaks at open-water and middle sites of the creek (1.85 and 1.04 mg m−3, respectively). Phytoplankton abundance followed a similar pattern to that of chlorophyll a with considerably higher abundance at these sites in May (3063.27 × 103 and 1082.34 × 103 individuals m−3, respectively). This unusually higher abundance was mostly due to the proliferation of the diatom Pseudo-nitzschia cf. delicatissima (Cleve) Heiden. Silicate concentrations were statistically significantly correlated with total phytoplankton. A total of 220 phytoplankton species were recorded during the study period (117 diatoms, 99 dinoflagellates and four cyanophytes). Diatoms dominated in the phytoplankton abundance (75%) and were followed by dinoflagellates (20%), while cyanophytes accounted for a minimal proportion. Of all phytoplankton species observed during the study, 21 diatom and four dinoflagellate species were considered as new records for the Red Sea, and two diatom and 14 dinoflagellate species were listed as harmful algal species worldwide.
Conclusion
The present study identifies a shifting pattern in the prevailing oligotrophic conditions of the Red Sea coastal waters. The major outcome of this study was the documentation of different phytoplankton species from the region, of which 21 diatom and four dinoflagellate species were considered as new records for the Red Sea. Another ecologically important aspect noted was the peculiar growth of the potentially harmful diatom species Pseudo-nitzschia cf. delicatissima. The higher density of this species observed in May was mainly due to the anthropogenic impact exerted on the system over a continuous period of time. Activities that alter the health status of the creek should be controlled and proper monitoring should be maintained in order to rejuvenate the system. Consequently, continuous monitoring of the coastal waters of the Red Sea is necessary to maintain a healthy ecosystem of ecological importance for the future generations.
Acknowledgements
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, Saudi Arabia, under the grant No. [G-462-150-40]. The authors, therefore, acknowledge with thanks DSR for technical and financial support. We extend our gratitude to Prof. Wafaaa Sallam for her critical reading of the manuscript and Mr. Reny P. Devassy for the assistance provided during the sampling process.
Author’s contribution
MMS conceived the idea of the project. MMS, MAA and AAK carried out the sampling and the analysis. MMS wrote the initial manuscript, MAA and AAK provided suggestions and recommendations. The authors declare that there is no conflict of interest regarding the publication of this article.
References
Abbass, S.G., Madkour, F.F. & Abu-El-Regal, M.A. (2018) Checklist of phytoplankton species in the Egyptian Red Sea Coast of Hurghada. Egypt. J. Aquat. Biol. & Fish. 22: 93–101.
Acker, J., Leptoukh, G., Shen, S., Zhu, T. & Kempler, S. (2008). Remotely-sensed chlorophyll a observations of the northern Red Sea indicate seasonal variability and influence of coastal reefs. J. Mar. Syst. 69 (3): 191–204. DOI: 10.1016/j.jmarsys.2005.12.006.
Al-Aidaroos, A.M., Devassy, R.P. & El-Sherbiny, M.M. (2019). Unusual dominance of harmful microalgae Pseudo-nitzschia delicatissima cf. (Cleve) Heiden in the coastal waters of Jeddah, central Red Sea. Pak. J. Bot. 51(2): 1–6. DOI: 10.30848/PJB2019-2(44).
Al-Amri, A.A., Qari, H.A. & El-Sherbiny, M.M. (2020). Distribution and community structure of microphytoplankton in relation to increasing anthropogenic impact along coastal waters of Jeddah, the central Red Sea. Oceanol. Hydrobiol. Stud. 49(2): 193–205. DOI: 10.1515/ohs-2020-0018.
Al-Farawati, R. (2010). Environmental conditions of the coastal waters of Southern Corniche, Jeddah, Eastern Red Sea: Physico-chemical approach. Aust. J. Basic Appl. Sci. 4: 3324–3337.
Al-Harbi, S.M. & Affan, M. (2016). Seasonal dynamics of epiphytic microalgae and their host seaweeds Florideophyceae at Jeddah coast, the Red Sea, Saudi Arabia. Pak. J. Bot. 48(3): 1289–1298.
Alsaafani, M.A., Alraddadi, T.M. & Albarakati, A.M. (2017). Seasonal variability of hydrographic structure in Sharm Obhur and water exchange with the Red Sea. Arab. J. Geosci. 10(14): 315. DOI: 10.1007/s12517-017-3108-8.
Banguera-Hinestroza, E., Eikrem, W., Mansour, H., Solberg, I., Cúrdia, J. et al. (2016). Seasonality and toxin production of Pyrodinium bahamense in a Red Sea lagoon. Harmful Algae 55: 163–171. DOI: 10.1016/j.hal.2016.03.002.
Basaham, A.S. & El-Sayed, M.A. (2006). Sharm Obhur: environmental consequences of 20 years of uncontrolled coastal urbanization. Marine Sciences 17(1).
Basaham, A.S. & El-Shater, A. (1994). Textural and mineralogical characteristics of the surficial sediments of Sharm Obhur, Red Sea coast of Saudi Arabia. Marine Sciences 5(1).
Bastos, L., Bio, A. & Iglesias, I. (2016). The importance of marine observatories and of RAIA in particular. Front. Mar. Sci. 3: 140. DOI: 10.3389/fmars.2016.00140.
Brierley, A.S. & Kingsford, M.J. (2009). Impacts of climate change on marine organisms and ecosystems. Curr. Biol. 19(14): R602–R614. DOI: 10.1016/j.cub.2009.05.046.
Clarke, K.R. & Gorley, R.N. (2006). PRIMER v6: User Manual/Tutorial. PRIMER-E, Plymouth.
Devassy, R.P., El-Sherbiny, M.M., Al-Sofyani, A.M. & Al-Aidaroos, A.M. (2017). Spatial variation in the phytoplankton standing stock and diversity in relation to the prevailing environmental conditions along the Saudi Arabian coast of the northern Red Sea. Mar. Biodivers. 47: 995–1008. DOI: 10.1007/s12526-017-0693-4.
Devassy, R.P., El-Sherbiny, M.M., Al-Sofyani, A.A., Crosby, M.P. & Al-Aidaroos, A.M. (2019). Seasonality and latitudinal variability in the diatom-cyanobacteria symbiotic relationships in the coastal waters of the Red Sea, Saudi Arabia. Symbiosis 78(3): 215–227.
Dowidar, N.M. (1983). The genus Ceratium from the Red Sea. J. Fac. Mar. Sci. 3: 5–37.
Dowidar, N., Raheem El-Din, S. & Aleem, A. (1978). Phytoplankton populations in the region of Obhur, Jeddah, Saudi Arabia. Bull. Fac. Sci., KAU 2: 271–292.
Edwards, F.J. (1987). Climate and oceanography. Red Sea 1: 45–68.
El-Sayed, M.A. (2002). Nitrogen and phosphorus in the effluent of a sewage treatment site on the eastern Red Sea coast: daily cycle, flux and impact on the coastal area. Int. J. Environ. Stud. 59(1): 73–94. DOI: 10.1080/00207230211959.
Gómez, F. (2013). Reinstatement of the dinoflagellate genus Tripos to replace Neoceratium, marine species of Ceratium (Dinophyceae, Alveolata). CICIMAR Oceánides 28: 1–22.
Hallegraeff, G.M. (2003). Harmful algal blooms: a global overview, Manual on harmful marine microalgae 33: 1–22.
Halpern, B.S., Walbridge, S., Selkoe, K.A., Kappel, C.V., Micheli, F. et al. (2008). A global map of human impact on marine ecosystems. Science 319: 948–952. DOI: 10.1126/science.1149345.
Hansen, P.J. (1991). Quantitative importance and trophic role of heterotrophic dinoflagellates in a coastal pelagic food web. Mar. Ecol. Prog. Ser. 73(2–3): 253–261.
Ismael, A.A. (2015). Phytoplankton of the Red Sea. In R. Najeeb, & I.C.F. Stewart (Eds.), The Red Sea: the formation, morphology, oceanography and environment of a young ocean basin (pp. 567–583). Springer: Berlin Heidelberg.
Kürten, B., Al-Aidaroos, A.M., Kürten, S., El-Sherbiny, M.M., Devassy, R.P. et al. (2016). Carbon and nitrogen stable isotope ratios of pelagic zooplankton elucidate ecohydrographic features in the oligotrophic Red Sea. Prog. Oceanogr. 140: 69–90. DOI: 10.1016/j.pocean.2015.11.003.
Kürten, B., Khomayis, H.S., Devassy, R., Audritz, S., Sommer, U. et al. (2015). Ecohydrographic constraints on biodiversity and distribution of phytoplankton and zooplankton in coral reefs of the Red Sea, Saudi Arabia. Mar. Ecol. 36(4): 1195–1214. DOI: 10.1111/maec.12224.
LeGresley, M. & McDermott, G. (2010). Counting chamber methods for quantitative phytoplankton analysis-haemocytometer, Palmer-Maloney cell and Sedgewick-Rafter cell. UNESCO (IOC Manuals and Guides, no 55) (IOC/2010/MG/55): 25–30.
Li, W., El-Askary, H., ManiKandan, K.P., Qurban, M.A., Garay, M.J. et al. (2017). Synergistic use of remote sensing and modeling to assess an anomalously high chlorophyll-a event during summer 2015 in the south central Red Sea. Remote Sens. 9(8): 778. DOI: 10.3390/rs9080778.
Madkour, F.F., El-Sherbiny M.M. & Aamer, M.A. (2010). Phytoplankton population along certain Egyptian coastal regions of the Red Sea. Egypt. J. Aquat. Biol. & Fish. 14(2): 95–109.
Mohamed, Z.A. & Al-Shehri, A.M. (2011). Occurrence and germination of dinoflagellate cysts in surface sediments from the Red Sea off the coasts of Saudi Arabia. Oceanologia 53(1): 121–136. DOI: 10.5697/oc.53-1.121.
Mohamed, Z.A. & Al-Shehri, A.M. (2012). The link between shrimp farm runoff and blooms of toxic Heterosigma akashiwo in Red Sea coastal waters. Oceanologia 54(2): 287–309. DOI: 10.5697/oc.54-2.287.
Mudarris, M.S.A. & Turki, A.J. (2006). Sewage water quality and its dilution in the coastal waters of South Corniche, Jeddah, Red Sea. JKAU: Met. Env. and Arid Land Agric. Sci. 17: 115–128.
Parsons, T.R., Maita, Y. & Lalli, C.M. (1984). A manual of chemical and biological methods for seawater analysis. Pergamon Press, Oxford.
Peña-García, D., Ladwig, N., Turki, A.J. & Mudarris, M.S. (2014). Input and dispersion of nutrients from the Jeddah Metropolitan Area, Red Sea. Mari. Poll. Bull. 80(1): 41–51. DOI: 10.1016/j.marpolbul.2014.01.052.
Qurban, M.A., Balala, A.C., Kumar, S., Bhavya, P.S. & Wafar, M. (2014). Primary production in the northern Red Sea. J. Mar. Syst. 132: 75–82. DOI: 10.1016/j.jmarsys.2014.01.006.
Racault, M.F., Raitsos, D.E., Berumen, M.L., Brewin, R.J., Platt, T. et al. (2015). Phytoplankton phenology indices in coral reef ecosystems: Application to ocean-colour observations in the Red Sea. Remote Sens. Environ. 160: 222–234. DOI: 10.1016/j.rse.2015.01.019.
Shaikh, E.A., Roff, J.C. & Dowidar, N.M. (1986). Phytoplankton ecology and production in the Red Sea off Jiddah, Saudi Arabia. Mar. Biol. 92(3): 405–416.
Taylor, F.J.R. (1976). Dinoflagellates from the International Indian Ocean Expedition. A report on material collected by the R.V. “Anton Bruun” 1963-1964. Bibl. Bot. 132: 1–234.
Tomas, C.R. (1997). Identifying Marine Phytoplankton. UNESCO. Protocols for the joint global ocean flux study (JGOFS), Manual and Guides 29, Academic press, USA.
Touliabah, H.E., Abu El-Kheir, W.S., Kuchari, M.G. & Abdulwass, N.I.H. (2010). Phytoplankton composition at Jeddah Coast-Red Sea, Saudi Arabia in relation to some ecological factors. JKAU: Mari. Sci. 22 (1): 115–131.
Wafar, M., Qurban, M.A., Ashraf, M., Manikandan, K.P., Flandez, A.V. et al. (2016). Patterns of distribution of inorganic nutrients in Red Sea and their implications to primary production. J. Mar. Syst. 156: 86–98. DOI: 10.1016/j.jmarsys.2015.12.003.