Paper category: Original research paper
Corresponding author: Khaled Gharbi (khaledgharbi10@yahoo.fr)
DOI: 10.2478/oandhs-2021-0033
Received: 02/05/2021
Accepted: 05/07/2021
Full text: here
Citation (APA style): Gharbi,K.,Fathalli,A.,Essid,R.,Fassatoui,C.,Romdhane,M.,Limam,F. & Rejeb Jenhani,A.(2021).Tunisian inland water microflora as a source of phycobiliproteins and biological activity with beneficial effects on human health. Oceanological and Hydrobiological Studies,50(4) 385-397. https://doi.org/10.2478/oandhs-2021-0033
Abstract
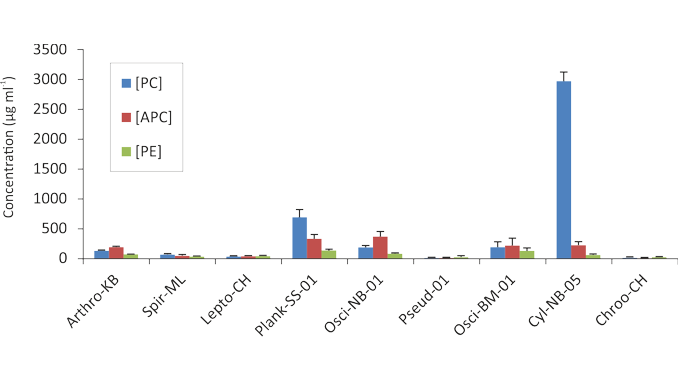
Ten monoclonal microalgal cultures were obtained from several Tunisian inland water bodies, and their dichloromethane and methanolic extracts were screened for antibacterial, antileishmanial, and antioxidant properties, as well as phycobiliprotein production capacity. Cylindrospermopsis raciborskii has been shown to synthesize high levels of phycocyanin and may be an effective alternative source to other sources used for commercial production of phycocyanin. Chroococcus sp. and Leptolyngbya sp1. exhibited the strongest radical scavenging activity against DPPH (IC50 = 212.15 and 263.91 μg ml−1, respectively), indicating their promising potential for use as new effective and non-toxic antioxidants. Furthermore, Dunaliella sp. showed an interesting antileishmanial activity against the pathogens Leishmania infantum and Leishmania major (IC50 = 151 and 284 µg ml−1, respectively), thus representing a good candidate for use against cutaneous and visceral leishmaniasis in Tunisia, a country endemic to these diseases where thousands of new cases are registered every year. These results suggest that the strains of microalgae featured in this work have the potential to serve as natural alternative, safe and sustainable sources of high value-added products that could be used to improve the final biomass value.
Conclusion
The present study provides important, clear and reliable information on 10 microalgal strains that colonize several Tunisian inland water bodies with respect to their potential for production of phycobiliproteins, and the evaluation of their antioxidant, antibacterial, and antileishmanial effects. The cyanobacterium Cylindrospermopsis raciborskii occupied a unique position among all the investigated isolates, considering its higher production and accumulation capacity of phycocyanin. This is a characteristic that certainly gives this strain a significant potential for use in the biotechnological production of this extremely valuable pigment. The genera Chroococcus and Leptolyngbya exhibited promising antioxidant activity, making them potential candidates as new natural sources of effective and non-toxic antioxidants. Eukaryotic unicellular green microalgae Dunaliella sp. showed the best antileishmanial properties. This result is of particular importance, especially in the context of Tunisia, where cutaneous leishmaniasis caused by L. major continues to cause thousands of new cases of infection each year and is a major health problem for the local population.
In view of the results obtained in the present work, we believe that the optimization of culture conditions, especially light and temperature, targeting the most potential strains will have an impact on increasing their potentiality. In addition, the use of genetic engineering as a measure to control the expression level of the genes encoding this activity would be of great benefit.
Acknowledgements
The present work was supported by Laboratoire Ecosystèmes et Ressources Aquatiques et Animales, Institut National Agronomique de Tunisie, University of Carthage with the collaboration of Bioactive Substances Laboratory, Biotechnology Center of Borj-Cedria, Tunisia, which are greatly acknowledged. This research was also financially supported by the Ministry of Higher Education and Scientific Research of the Tunisian government.
References
Agyei, D., Danquah, M.K., Sarethy, I.P. & Pan, S. (2015). Antioxidative peptides derived from food proteins. In: Rani V, Yadav U (eds) Free radicals in human health and disease. Springer, New Delhi. Retrieved November 30, 2020, from SpringerLink https://link.springer.com/chapter/citeas. DOI:10.1007/978-81-322-2035-0_26.
Anonymous, World Health Organisation, Eastern Mediterranean Regional office (WHO EMRO). (2017). Cutaneous leishmaniasis in Tunisia. Retrieved May 16, 2020, from https://www.who.int/leishmaniasis/ burden/Leishmaniasis_Tunisia/en/.
Askun, T., Tekwu, E.M., Satil, F., Modanlioglu, S. & Aydeniz, H. (2013). Preliminary antimycobacterial study on selected Turkish plants (Lamiaceae) against Mycobacterium tuberculosis and search for some phenolic constituents. BMC Complement Altern. Med. 13: 365. DOI: 10.1186/1472-6882-13-365.
Babic, O., Kovac, D., Raseta, M., Sibul, F., Svircev, Z. et al. (2016). Evaluation of antioxidant activity and phenolic profile of filamentous terrestrial cyanobacterial strains isolated from forest ecosystem. J. Appl. Phycol. 28(4): 2333–2342. DOI:10.1007/s10811-015-0773-4.
Bachchhav, M.B., Kulkarni, M.V., & Ingale, A.G. (2016). Enhanced phycocyanin production from Spirulina platensis using Light Emitting Diode. J. Inst. Eng. India Ser. E. 98(1): 41–45. DOI: 10.1007/s40034-016-0090-8.
Basheva, D., Moten, D., Stoyanov, P., Belkinova, D., Mladenov, R. et al. (2018). Content of phycoerythrin, phycocyanin, alophycocyanin and phycoerythrocyanin in some cyanobacterial strains: Applications. Eng. Life Sci. 18(11): 861–866. DOI: 10.1002/elsc.201800035.
Bennett, A. & Bogorod, L. (1973). Comple-mentary chromatic adaptation in filamentous blue−green alga. J. Cell. Biol. 58(2): 419−435. DOI: 10.1083/jcb.58.2.419.
Bhagavathy, S., Sumathi, P. & Jancy Sherene Bell, I. (2011). Green algae Chlorococcum humicola - a new source of bioactive compounds with antimicrobial activity. Asian Pac. J. Trop. Biomed. 1(1): S1–S7. DOI: 10.1016/S2221-1691(11)60111-1.
Blagojevic, D., Babic, O.B., Raseta, M., Sibul, F.S., Janjusevic, L. et al. (2018). Antioxidant activity and phenolic profile in filamentous cyanobacteria: the impact of nitrogen. J. Appl. Phycol. 30(4): 2337–2346. DOI: 10.1007/s10811-018-1476-4.
Blancheton, A. (1985). Production d’Algues unicellulaires. Ifremer report, Ifremer Bibliothèque de PALAVAS, https://archimer.ifremer.fr/doc/00000/1749/.
Bourrelly, P. (1966). Les algues d’eau douce : les algues vertes (Vol. 1). N. Boubée & Cie (Ed.), Paris, France.
Campino, L. & Maia, C. (2010). Epidemiologia das leishmanioses em Portugal (In Portuguese) (English summary). Acta Méd. Port. 23(5): 859–864.
Campino, L., Pratlong, F., Abranches, P., Rioux, J.A., Santos-Gomes, G. et al. (2006). Leishmaniasis in Portugal: Enzymatic Polymorphism of Leishmania infantum based on Identification of 213 Strains. Trop. Med. Int. Health. 11(11): 1708–1714. DOI: 10.1111/j.1365-3156.2006.01728.x.
Celiktas, O.Y., Kocabas, E.E.H, Bedir, E., Sukan, F.V., Ozek, T. et al. (2007). Antimicrobial activities of methanol extracts and essential oils of Rosmarinus officinalis, depending on location and seasonal variations. Food Chem. 100(2): 553–559. DOI: 10.1016/j.foodchem.2005.10.011.
Cepoi, L., Rudi, L., Miscu, V., Cojocari, A., Chiriac, T. et al. (2009). Antioxidative activity of ethanol extracts from Spirulina platensis and Nostoc linckia measured by various methods. Analele Univ. din Oradea, Fasc. Biol. 16: 43–48.
Cheel, C.J., Theoduloz, C., Rodriguez, J.A., Caligari, P.D.S. & Schmeda-Hirschmann G. (2007). Free radical scavenging activity and phenolic content in achenes and thalamus from Fragariachiloensis ssp. chiloensis, F. vesca and F. x ananassa cv Chandler. Food Chem. 102(1): 36–44. DOI: 10.1016/j.foodchem.2006.04.036.
De Oliveira, C.A., Oliveira, W.C., Ribeiro, S.M.R., Stringheta, P.C. & Nascimento, A.G. (2014). Effect of light intensity on the production of pigments in Nostoc spp. Euro. J. of Biol. and Med. Sci. Res. 2(1): 23–36.
Dewanto, V.W.X., Adom, K.K. & Liu, R.H. (2002). Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 50(10): 3010–3014. DOI: 10.1021/jf0115589.
Essid, R., Rahali, F.Z., Msaadaa, K., Sghair, I., Hammami, M. et al. (2015). Antileishmanial and cytotoxic potential of essential oils from medicinal plants in Northern Tunisia. Ind. Crops Prod. 77: 795–802. DOI: 10.1016/j.indcrop.2015.09.049.
Fathalli, A., Jenhani, A.B., Moreira, C., Welker, M., Romdhane, M. et al. (2011). Molecular and phylogenetic characterization of potentially toxic cyanobacteria in Tunisian freshwaters. Syst. Appl. Microbiol. 34(4): 303–310. DOI: 10.1016/j.syapm.2010.12.003.
Geitler, L. (1932). In Rabenhorst’s, Koeltz (Ed.), Cyanophyceae von Europa in Kryptogamen-Flora. Scientific Books. Koeniggstein W, Germany.
Goiris, K., Muylaert, K., Fraeye, I., Foubert, I., Brabanter, J.D. et al. (2012). Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J. Appl. Phycol. 24(6): 1477–1486. DOI: 10.1007/s10811-012-9804-6.
Heimler, D., Vignolini, P., Giulia, D.M., Vincieri, F.F. & Romani, A. (2006). Anti-radical activity and polyphenol composition of local Brassicaceae edible varieties, Food Chem. 99(3): 464–469. DOI: 10.1016/j.foodchem.2005.07.057.
Hossain, M.F., Ratnayake, R.R., Meerajini, K. & Wasantha Kumara, K.L. (2016). Antioxidant properties in some selected cyanobacteria isolated from fresh water bodies of Sri Lanka. Food Sci. Nutr. 4(5): 753–758. DOI: 10.1002/fsn3.340.
Horvath, H., Kovacs, A.W., Riddick, C.A.L. & Présing, M. (2013). Extraction methods for phycocyanin determination in freshwater filamentous cyanobacteria and their application in a shallow lake. Eur. J. Phycol. 48(3): 278–286. DOI: 10.1080/09670262.2013.821525.
Ijaz, S. & Hasnain, S. (2016). Antioxidant potential of indigenous cyanobacterial strains in relation with their phenolic and flavonoid contents. Nat. Prod. Res. 30(11): 1297–1300. DOI: 10.1080/14786419.2015.1053088.
Jalal, K.C.A., Shamsuddin, A.A., Rahman, M.F., Nurzatul, N.Z. & Rozihan, M. (2013). Growth and Total Carotenoid, Chlorophyll a and Chlorophyll b of Tropical Microalgae (Isochrysis sp.) in Laboratory Cultured Conditions. J. Biol. Sci. 13(1): 10–17. DOI: 10.3923/jbs.2013.10.17.
Kang, H.K., Seo, C.H. & Park, Y. (2015). Marine peptides and their anti-infective activities. Mar. Drugs 13(1): 618–654. DOI: 10.3390/md13010618.
Kawamura, N.C., Hirahashi, T., Nagai, T., Yamada, H., Katoh, T. et al. (2004). Phycocyanin enhances secretary IgA antibody response and suppresses allergic IgE antibody response in mice immunized with antigen-entrapped biodegradable microparticles. J. Nutr. Sci. Vitaminol. 50(2): 129–136. DOI: 10.3177/jnsv.50.129.
Komárek, J., Anagnostidis, K. (1999). Cyanoprokaryota, Part 1: Chroococcales, Su¨ sswasserflora von Mitteleuropa, Bd 19/1. Spektrum Akademischer Verlag.
Komárek, J., Anagnostidis, K. (2005). Cyanoprokaryota, Part 2: Oscillatoriales, Su¨ sswasserflora von Mitteleuropa, Bd 19/2. Spektrum Akademischer Verlag.
Lamers, P.P., Van de Laak, C.C., Kaasenbrood, P.S., Lorier, J., Janssen, M. et al. (2010). Carotenoid and fatty acid metabolism in light-stressed Dunaliella salina. Biotechnol. Bioeng. 106(4): 638–648. DOI: 10.1002/bit.22725.
Lanigan, R.S. & Yamarik, T.A. (2002). Final report on the safety assessment of BHT. Int. J. Toxicol. 2: 19–94. DOI: 10.1080/10915810290096513.
Liu, Y., Xu, L., Cheng, N., Lin, L. & Zhang, C. (2000). Inhibitory effect of phycocyanin from Spirulina platensis on the growth ofhuman leukemia K562 cells. J. Appl. Phycol. 12(2): 125–130. DOI: 10.1023/A:1008132210772.
Maadane, A., Merghoub, N., Ainane, T., El Arroussi, H., Benhima, R. et al. (2015). Antioxidant activity of some Moroccan marine microalgae: pufa profiles, carotenoids and phenolic content. J. Biotechnol. 215: 13–19. DOI: 10.1016/j.jbiotec.2015.06.400.
Maadane, A., Merghoub, N., Mernissi, N., Ainane, T., Amzazi, S. et al. (2017). Antimicrobial activity of marine microalgae isolated from Moroccan coastlines. J. Microbiol. Biotechnol. Food Sci. 6(6): 1257–1260. DOI: 10.15414/jmbfs.2017.6.6.1250-1256.
Ngo, D.H., Wijesekara, I., Vo, T.S., Van, T.Q. & Kim, S.K. (2011). Marine food-derived functional ingredients as potential antioxidants in the food industry: An overview. J. Appl. Phycol. 44(2): 523–529. DOI: 10.1016/j.foodres.2010.12.030.
Olmos, J., Paniagua, J. & Contreras, R. (2000). Molecular identification of Dunaliella sp. utilizing the 18s rDNA gene. Lett. Appl. Microbiol. 30(1): 80–84. DOI: 10.1046/j.1472-765x.2000.00672.x.
Penton-Rol, G., Marín-Prida, J., Pardo-Andreu, G., Martínez-Sánchez, G., Acosta-Medina, E.F. et al. (2011). C-Phycocyanin is neuroprotective against global cerebral ischemia/reperfusion injury in gerbils. Brain Res. Bull. 86(1–2): 42–52. DOI: 10.1016/j.brainresbull.2011.05.016.
Pereira, H., Custódio L., Rodrigues, M.J., De Sousa, C.B., Oliveira, M. et al. (2015). Biological Activities and Chemical Composition of Methanolic Extracts of Selected Autochthonous Microalgae Strains from the Red Sea. Mar. Drugs 13(6): 3531–3549. DOI: 10.3390/md13063531.
Rimbau, V., Camins, A., Pubill, D., Sureda, F.X., Romay, C. et al. (2001). C-phycocyanin protects cerebellar granule cells from low potassium/serum deprivation-induced apoptosis. Naunyn Schmiedebergs Arch. Pharmacol. 364(2): 96–104. DOI: 10.1007/s002100100437.
Rippka, R., Derueles, J., Waterbury, J.B., Herdman, M. & Stanier R.Y. (1979). Generic Assignments, Strain Histories and Properties of Pure Cultures of Cyanobacteria. J. Gen. Microbiol. 111(1): 1–61. DOI: 10.1099/00221287-111-1-1.
Safafar, H., Van Wagenen, J., Møller, P. & Jacobsen, C. (2015). Carotenoids, Phenolic Compounds and Tocopherols Contribute to the Antioxidative Properties of Some Microalgae Species Grown on Industrial Wastewater. Mar. Drugs 13(12): 7339–7356. DOI: 10.3390/md13127069.
Sanchez, L.M., Lopez, D., Vesely, B.A., Della Togna, G., Gerwick, W.H. et al. (2010). Almiramides A-C: discovery and development of a new class of leishmaniasis lead compounds. J. Med. Chem. 53(10): 4187–4197. DOI: 10.1021/jm100265s.
Singh, D.P, Prabha, R., Verma, S., Meena, K.K. & Yandigeri, M. (2017). Antioxidant properties and polyphenolic content in terrestrial cyanobacteria. 3 Biotech. 7(2): 134. DOI: 10.1007/s13205-017-0786-6.
Takano, H., Arai, T., Hirano, M. & Matsunaga, T. (1995). Effects of intensity and quality of light on phycocyanin production by a marine cyanobacterium Synechococcus sp. NKBG 042902. Appl. Microbiol. Biotechnol. 43: 1014–1018. DOI: 10.1007/BF00166918.
Yang, D.J., Lin, J.T., Chen, Y.C., Liu, S.C., Lu, F.J. et al. (2013). Suppressive effect of carotenoid extract of Dunaliella salina alga on production of LPS-stimulated pro-inflammatory mediators in RAW264.7 cells via NF-jB and JNK inactivation. J. Funct. Foods. 5(2): 607–615. DOI: 10.1016/j.jff.2013.01.001.
