Paper category: Original research paper
Corresponding author: Abdelkarim Derbali (derbali10@gmail.com)
DOI: 10.1515/ohs-2021-0012
Received: 27/10/2020
Accepted: 17/11/2020
Full text: here
Citation (APA style): Derbali,A. & Jarboui,O.(2021).Stock mapping, size structure and biological parameters of the clam Polititapes aureus in the shellfish production area of the southern Tunisian waters (Central Mediterranean). Oceanological and Hydrobiological Studies,50(2) 128-136. https://doi.org/10.2478/oandhs-2021-0012
Abstract
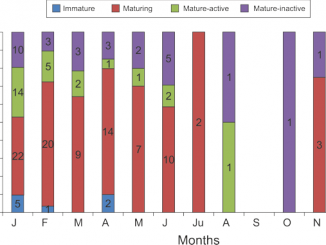
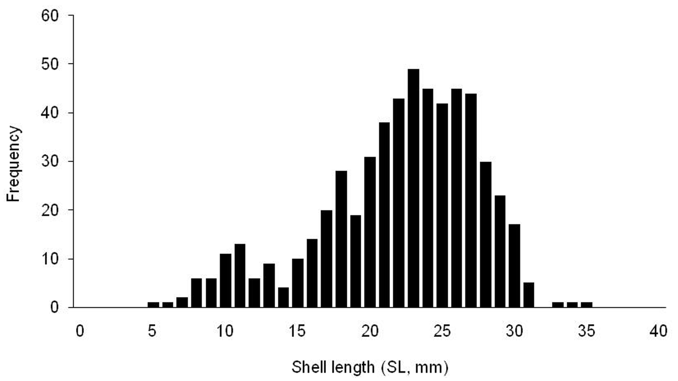
The clam Polititapes aureus is one of the most abundant shellfish species in the southern Tunisian waters. Its current exploitation status and management are becoming a major concern for fishing industry in Tunisia. The significant ecological role of the species and possible future commercial benefits require a better knowledge of its stock. This research is the first attempt to investigate its current status in an area with the largest shellfish production. The obtained results showed a scattered distribution. The stock density ranged from 0 to 124 ind. m<sup>−2</sup>, and biomass values varied from 0 to 300 g m<sup>−2</sup>. This results in a remarkable biomass of 201.2 (± 64.6) t and high abundance reaching 91.3 ± 32.9 million individuals, estimated an area of 4182 ha. The species distribution was also investigated, with the size ranging from 4.9 to 35.34 mm. The overall sex ratio (F:M) was 1.26:1, which significantly deviated different from parity (1:1). The main environmental factors were identified and several abiotic parameters were found to strongly affect the spread of the clam species. The clam reproduce well and is expected to almost meet the domestic market demand through artisanal fishery.
Acknowledgements
This work was carried out as part of the research at the Laboratory of Fisheries Sciences of the National Institute of Marine Sciences and Technologies (INSTM). We wish to thank the anonymous reviewers for their suggestions and constructive comments, which helped to improve the quality of this manuscript.
References
Akester, R.J. & Martel, A.L. (2000). Shell shape, dysodont tooth morphology, and hinge-ligament thickness in the bay mussel Mytilus trossulus correlate with wave exposure. Can. J. Zool. 78(2): 240–253. DOI: 10.1139/cjz-78-2-240.
Ben Mustapha, K. & Hattour, A. (2013). Le couvert végétal marin du golfe de Gabès: cartographie et réseau de surveillance de l’herbier de Posidonie. Tunisia. Bull. Inst. Natn. Sci. Tech. Mer. 164 pp.
Ben Othman, S. (1973). The south of Tunisia (Gulf of Gabes), hydrology, sedimentology, flora and fauna. Unpublished doctoral dissertation, University of Tunis, Tunisia. (In French).
Ben Salem, S., Franquesa, R. & El Abed, A. (2002). Socio-economic indicators for fishing in the Gulf of Gabes (Tunisia). INSTM and FAO-Copemed, 34 pp. (In French).
Beukema, J.J. & Meehan, B.W. (1985). Latitudinal variation in linear growth and other shell characteristics of Macoma balthica. Mar. Biol. 90: 27–33. DOI: 10.1007/BF00428211.
Buchanan, J.B. (1984). Sediment analysis. In N.A. Holme & A.D. McIntyre (Eds.), Methods for the study of marine benthos (pp. 41–65). Oxford: Blackwell Scientific Publications.
Bustamante, R.H., Branch, G.M., Eekhout, S., Robertson, B., Zoutendyk, P. et al. (1995). Gradients of intertidal primary productivity around the coast of South Africa and their relationships with consumer biomass. Oecologia 102: 189–201.
Chamtouri, I., Abida, H., Khanfir, H. & Bouri, S. (2008). Impacts of at-site wastewater disposal systems on the groundwater aquifer in arid regions: case of Sfax city, Southern Tunisia. Environ. Geol. 55: 1123–1133. DOI: 10.1007/s00254-007-1060-8.
Chintiroglou, C.C., Antoniadou, C. & Damianidis, P. (2000). Spatial dispersion and density of the Paranemonia vouliagmeniensis population in Vouliagmeni Lagoon. J. Mar. Biol. Ass. U. K. 80: 941–942. DOI: 10.1017/S0025315400002939.
Claxton, W.T., Wilson, A., Mackie, G.L. & Boulding, E.G. (1998). A genetic and morphological comparison of shallow and deepwater populations of the introduced dreissenid bivalve Dreissena bugensis. Can. J. Zool. 76(7): 1269–1276. DOI: 10.1139/cjz-76-7-1269.
da Costa, F., Aranda-Burgos, J.A., Cerviño-Otero, A., Fernández-Pardo, A., Louzán, A. et al. (2013). Clam reproduction. In F. da Costa Gonzáles (Eds.), Clam fisheries and aquaculture (pp. 45–71). New York: Nova Publishers.
Derbali, A. (2006). Contribution to the study of abundance and the spatial distribution of some bivalves species in littoral zone of the Boughrara Lagoon. Unpublished master thesis, University of Sfax, Tunisia. (In French).
Derbali, A. (2011). Biology, abundance and cartography of two bivalves species: the pearl-oyster Pinctada radiata and the cockle Cerastoderma glaucum in the Gulf of Gabes. Unpublished doctoral dissertation, University of Sfax, Tunisia. (in French).
Derbali, A., Jarboui, O. & Ghorbel, M. (2009a). Reproductive biology of the cockle Cerastoderma glaucum (Mollusca: Bivalvia) from the north coast of Sfax (Gulf of Gabes, Tunisia). Cienc. Mar. 35(2): 141–152.
Derbali, A., Jarboui, O. & Ghorbel, M. (2011). Distribution, abundance and population structure of the pearl oyster Pinctada radiata (Mollusca: Bivalvia) in southern Tunisian waters (Central Mediterranean). Cah. Biol. Mar. 52(1): 23–31.
Derbali, A., Jarboui, O., Ghorbel, M. & Dhieb, K. (2009b). Reproductive biology of the pearl oyster, Pinctada radiata (Mollusca: Pteriidae), in northern Kerkennah Island (Gulf of Gabes). Cah. Biol. Mar. 50: 215–222.
Derbali, A., Elhasni, K., Jarboui, O. & Ghorbel, M. (2012). Distribution, abundance and biological parameters of Cerastoderma glaucum (Mollusca: Bivalvia) along the Gabes coasts (Tunisia south, Central Mediterranean). Acta Adriat. 53(3): 363–374.
Derbali, A., Hadj Taieb, A., Kammoun, W., Jarboui, O. & Ghorbel, M. (2014). Mapping stocks and population structure of the cockle Cerastoderma glaucum in the littoral zone of Sfax (Tunisia, Central Mediterranean). Cah. Biol. Mar. 55: 353–361.
Derbali, A, Hadj Taieb, A., Kammoun, W., Gouirah, J., Ouannes-Ghorbel, A. et al. (2016). Stock assessment, spatial distribution and biological parameters of the clam Venerupis decussata along the Sfax coasts (Tunisia, Central Mediterranean). J. Mar. Biol. Ass. U. K. 96(1): 177–184.
Dridi, S., Romdhane, M.S. & Elcafsi, M. (2007). Seasonal variation in weight and biochemical composition of the Pacific oyster, Crassostrea gigas in relation to the gametogenic cycle and environmental conditions of the Bizerte lagoon, Tunisia. Aquaculture 263(1–4): 238–248. DOI: 10.1016/j.aquaculture.2006.10.028.
Drummond, L., Mulcahy, M. & Culloty, S. (2006). The
reproductive biology of the Manila clam, Ruditapes philippinarum, from the North-West of Ireland. Aquaculture 254(1–4): 326–340. DOI: 10.1016/j.aquaculture.2005.10.052.
El-Shabrawy, G.M. (2001). Ecological studies on macrobenthos of Lake Qarun, El-Fayoum, Egypt. J. Egypt. Acad. Soc. Environ. Dev. 1: 29–49.
Enríquez-Díaz, M., Pouvreau, S., Chávez-Villalba, J. & Le Pennec, M. (2009). Gametogenesis, reproductive investment, and spawning behavior of the Pacific giant oyster Crassostrea gigas: evidence of an environment-dependent strategy. Aquac. Int. 17(491): 491–506. DOI: 10.1007/s10499-008-9219-1.
Fishar, M.R.A. (2000). Long-term changes (1974–1996) of benthic macroinvertebrates in Lake Qarun (Faiyoum – Egypt). Egypt. J. Aquat. Biol. Fish. 4: 61–73.
Fouda, M.M. & Abou-Zeid, M.M. (1990). Bivalves of the Suez Canal lakes. Proc. Zool. Soc. Egypt 21: 231–240.
Franz, D.R. (1993). Allometry of shell and body weight in relation to shore level in the intertidal bivalve Geukensia demissa (Bivalvia: Mytilidae). J. Exp. Mar. Biol. Ecol. 174(2): 193-207. DOI: 10.1016/0022-0981(93)90017-I.
Fuiman, L.A., Gage, J.D. & Lamont, P.A. (1999). Shell morphometry of the deep sea protobranch bivalve Ledella pustulosa in the Rockall Trough, North-East Atlantic. J. Mar. Biol. Ass. U. K. 79(4): 661–671. DOI: 10.1017/S0025315498000824.
Gabbott, P.A. (1983). Development and seasonal metabolic activities in marine mollusks. In P.W. Hochachka & K.M. Wilbur (Eds.), The Mollusca (pp. 165–277). Environmental physiology and biochemistry (Vol. 2). New York: Academic Press.
Gulland, J.A. (1969). Handbook of the evaluation methods of the aquatic animals stocks. First part: Analysis of populations). Man. FAO Sci. Halieut., 4: 160 pp. (In French).
Hinch, S.G. & Bailey, R.C. (1988). Within- and among-lake variation in shell morphology of the freshwater clam Elliptio complanata (Bivalvia: Unionidae) from south-central Ontario lakes. Hydrobiologia 157: 27–32.
Jara-Jara, R., Pazos, A.J., Abad, M., García-Martín, L.O. & Sánchez, J.L. (1997). Growth of clam seed (Ruditapes decussatus) reared in the wastewater effluent from a fish farm in Galicia (NW Spain). Aquaculture 158(3–4): 247–262. DOI: 10.1016/S0044-8486(97)00196-8.
Kandeel, K.E. (2017). Invasion dynamics of a non-indigenous bivalve clam; Venerupis aurea (Gmelin, 1971), in Lake Qarun, Egypt. J. Adv. Biol. 10(2): 2056–2066.
Laing, I., Utting, S.D. & Kilada, R.W.S. (1987). Interactive effect of diet and temperature on the growth of juvenile clams. J. Exp. Mar. Biol. Ecol. 113(1): 23–38.
Lubet, P. (1959). Research on the sexual cycle and the gametes emission at the Mytilidae and the Pectenidae (bivalves molluscs). Rev. Trav. Inst. Pech. Marit. 23: 387–548. (In French).
Lucas, A. (1965). Research on the sexuality of Bivalve Molluscs. Bull. Biol. Fr. Belg. 95: 115–247. (In French).
Menge, B.A. & Olson, A.M. (1990). Role of scale and environmental factors in regulation of community. Trends Ecol. Evol. 5(2): 52–57. DOI: 10.1016/0169-5347(90)90048-I.
Mzighani, S. (2005). Fecundity and population structure of cockles, Anadara antiquata L. 1758 (Bivalvia: Arcidae) from a sandy/muddy beach near Dar es Salaam, Tanzania. West Indian Ocean J. Mar. Sci. 4(1): 77–84. DOI: 10.4314/wiojms.v4i1.28475.
Newell, C.R. & Hidu, H. (1982). The effects of sediment type on growth rate and shell allometry in the soft shelled clam Mya arenaria (L). J. Exp. Mar. Biol. Ecol. 65(3): 285–295. DOI: 10.1016/0022-0981(82)90060-0.
Sobral, P. & Widdows, J. (2014). Effects of elevated
temperatures on the scope for growth and resistance to air exposure of the clam Ruditapes decussatus (L.), from southern Portugal. Sci. Mar. 61(2): 163–171.
Stergiou, K.I., Christou, E.D., Georgopoulos, D., Zenetos, A. & Souvermezoglou, C. (1997). The Hellenic Seas: physics, chemistry, biology and fisheries. Oceanogr. Mar. Biol. 35: 415–538.
Tlig-Zouari, S., Rabahoui, L., Irathni, I. & Ben Hassine, O.K. (2009). Distribution, habitat and population densities of the invasive species pinctata radiata (Mollusca: Bivalvia) along the northern and eastern coasts of Tunisia. Cah. Biol. Mar. 50: 131–142.
Tlig-Zouari, S., Rabaoui, L., Irathni, I., Diawara, M. & Ben Hassine, O.K. (2010). Comparative morphometric study of the invasive pearl oyster Pinctada radiata along the Tunisian coastline. Biologia 65(2): 294–300. DOI: 10.2478/s11756-010-0023-9.
Vilela, H. (1950). Benthic life of Tapes decussatus. Trav. St. Biol. Mar. Lisbonne 53: 1–79. (In Portuguese).
Zar, J.H. (1996). Biostatistical analysis, 3rd edn. Englewood Cliffs, NJ: Prentice-Hall.